Il Farmaco Volume 57, Issue 10 , 24 September 2002, Pages 853-859DOI:
10.1016/S0014-827X(02)01276-4
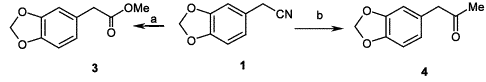 Synthesis of 1,3-benzodioxole-5-acetic acid methyl ester
Synthesis of 1,3-benzodioxole-5-acetic acid methyl ester1,3-Benzodioxole-5-acetonitrile (
1) (2 g, 12.4 mmol) was dissolved in a freshly prepared solution of MeOH/MeCOCl (1/1, v/v, 60 ml) and the reaction mixture was stirred at 0°C for 8 h. The solvent was removed under vacuum and the residue was treated with water (60 ml) and extracted with ethyl ether (2×80 ml). The pooled organic extracts were dried (Na
2SO
4) and the solvent was evaporated at reduced pressure to yield 1,3-benzodioxole-5-acetic acid methyl ester (2.3 g, 95%) as an oil.
Synthesis of 1-(1,3-benzodioxol-5-yl)-2-propanone (MDP2P)To a stirred solution of 1,3-Benzodioxole-5-acetonitrile (
1) (4 g, 24.8 mmol) and methyl magnesium bromide (3.0 M solution in THF, 9.5 ml, 28.5 mmol), in THF (80 ml) was added CuBr (64 mg, 0.43 mmol), and the mixture was refluxed under nitrogen for 30 min. After cooling to 0–5°C, 5 ml of H
2O was cautiously added, followed by 30 ml of 15% H
2SO
4. After stirring for 14 h, 60 ml of ether was added, the phases were separated, and the aqueous layer was extracted twice more with 50 ml portions of ether. The combined organic phase was dried (Na
2SO
4) and the solvent removed under reduced pressure to afford 1-(1,3-benzodioxol-5-yl)-2-propanone (MDP2P) (yield 30%, 1.32 g) as a light brown oil.
Rf (cyclohexane/EtOAc, 60/40) 0.56
1H NMR: 2.14 (s, 3H, CH
3), 3.59 (s, 2H, CH
2), 5.93 (s, 2H, OCH
2O), 6.61–6.77 (m, 3H, Ar).