Off course diethylamine adds on the double bond of methyl vinyl ketone yielding Et2N-CH2-CH2-CO-CH3 but I said "nuclephylic" while methyl vinyl ketone is an electrophyle in this reaction.
There are ketones that can bee nucleophylic enough for the addition on 2-nitropropene. 1,3-diketones for example, their deprotonated form actually, and they don't even need a basic catalyst. Therefore, even better than these O-TMS masked ketones (SnCl4 is a Lewis acid for 2-nitropropene activation). But I said "plain ketones", meaning simple ones like cyclohexanone in our case.
You can see that benzalacetone synthesis requires more drastic conditions. That is exactly because acetone is not nucleophylic enough. Only its enolate is.
Therefore, I guess if you make a cyclohexy Grignard you can then add it to CH2=CH(NO2)-Me and obtain the wanted 1-cyclohexyl-2-nitropropane. But cyclohexene does not have a chance. (Though if you go trough the trouble of preparing the Grignard you might make some phenyl Grignard and directly get to 1-phenyl-2-nitropropane.)
EDIT:WizardX, may I propose an alternative to your nice idea of last step dehydrogenation to form the amphetamine’s phenyl? I like the idea because it is so un-orthodox, one of those that usually come to mind only as the last possibility.
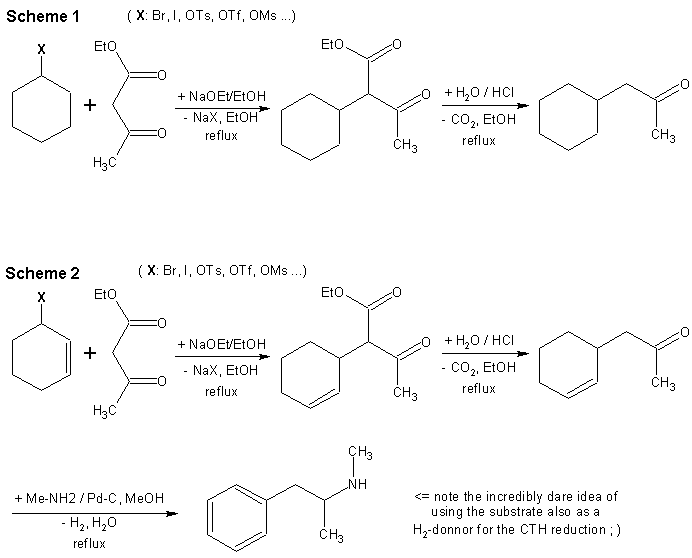
Scheme 1.
The alkylations of acetoacetates and other 1,3-dicarbonyl compounds are straightforward. I don’t have any example of this specific preparation at hand but I found an example of 65% yield when using izobutylbromide in the Organikum book and 50% yield with isopropyl iodide. The yields with these secondary halides are however lower than with the primary ones due to the concurrent HX elimination reaction or steric hindrance. Cyclohexyl-X can bee of course prepared from cyclohexanol by one of the many methods known at The Hive: the KI/H3PO4 method; alcohol bromination with HBr; esterification with TsOH …
The hydrolysis/decarboxylation step is very simple and can bee done either in one (reflux in 20% HCl until the CO2 effervescence ends – few hours) or two steps (hydrolysis with NaOH and decarboxylative vacuum distillation).
The amination of this ketone can bee done by most of the same methods that also proved successful for P2P, following by the known dehydrogenation to obtain the wanted product.
Scheme 2I could not help myself proposing a more funny way to meth. The first and second steps are analogous to those in scheme 1 except for an allylic (pseudo)halogenide like cyclohex-3-yl-bromide being used. Just as a comparative example, Organikum reports a yield of 85% when using allylbromide for acetoacetates alkylation.
The only true difference is that the amination and dehydrogenation are done in the same step. This is based on the cyclohexene rings being excellent hydrogen donors for CTH reductions as well as yielding benzene ring in the same time. Well, you don’t have to take this one too seriously but it looks cool nevertheless.