A Convenient Reductive Deamination (Hydrodeamination) of Aromatic AminesYapin Wang and Frank S. Guziec Jr.,
J. Org. Chem., 66, 8293-8296 (2001)
AbstractReductive deamination (hydrodeamination) of aromatic amines can be conveniently carried out by amination of the corresponding arylamine methanesulfonamides using chloroamine under alkaline conditions. The intermediate aryl methanesulfonylhydrazines directly eliminate methanesulfinic acid, affording diazenes which extrude nitrogen affording the desired deaminated products. Both sulfonamide formation and reduction reactions occur in high yield and are compatible with a variety of functional groups.Reductive removal of an amine group, hydrodeamination, is a very important transformation in organic chemistry. Reactions involving such reductive processes are particularly useful in aromatic chemistry because of the strong directing effects associated with amine substituents. Generally, reductive deamination is carried out on arylamines by diazotization of the amine function, affording the corresponding diazonium salt followed by reduction using hypophosphorous acid, sodium borohydride, or a variety of other reducing agents.
1 Alternative deamination procedures are very limited in the literature.
In our earlier work, we had investigated the use of chloroamine as an aminating agent.
3 Although this reagent proved to be extremely effective in the amination reactions, the usual preparation of chloroamine was not convenient and potentially hazardous. In this procedure sodium hypochlorite is added to a cooled solution of aqueous ammonia, and the resulting gaseous chloroamine distilled at 40-45°C under reduced pressure into a dry ice-cooled ether solution. Ethereal chloroamine could be obtained in about 30% yield.
11 An alternative less well-known published procedure for the preparation of chloroamine proved to be much more convenient.
12 This procedure involves buffering the sodium hypochlorite-ammonia reaction with ammonium chloride, avoiding over-chlorination of the initially formed chloroamine which can be readily extracted in organic solvents. This reaction does not require distillation of the ether-chloroamine mixture and affords ethereal chloroamine in typical yields of greater than 90%.
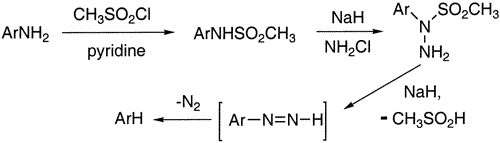 Experimental Section
Experimental Section
Chloroamine (Modification of the Procedure of Schmitz and Co-Workers)12
Ammonium chloride (0.707 g, 13.2 mmol) was added to a 2.0 M aqueous ammonia solution (4.35 mL, 8.7 mmol), and the mixture was cooled in an ice bath. To this cooled mixture was added dropwise 5.25% (0.76 M) aqueous sodium hypochlorite (commercial bleach) (18.8 mL, 14.3 mmol) over 15 min and the mixture stirred an additional 15 min at 0°C. The aqueous solution was extracted with a cold (0°C) mixture of dichloromethane (12 mL) and ether (10 mL) and then with another portion of cold dichloromethane (12 mL). The combined organic extracts were dried with CaCl2 at -10°C for 30 min. An aliquot of the ethereal chloroamine solution could be iodometrically titrated using potassium iodide and sodium thiosulfate to accurately determine the chloroamine concentration in the organic solution (see Supporting Information). The typical yield of chloroamine in solution using this procedure was 5.0 mmol. Note: Although no problems were experienced in the preparation and handling of chloroamine using this procedure, it should be noted that only dilute solutions of chloroamine should be prepared. More concentrated solutions readily decompose potentially creating a safety hazard.14 The preparation and reactions of chloroamine should also be carried out in a well-ventilated hood.
Arylamine Hydrodeamination; General Procedure
Methanesulfonyl chloride (0.92 mL, 12 mmol) was added to a cooled 0°C stirred solution of the arylamine (12 mmol) in pyridine (1.09 mL) and dichloromethane (35 mL), keeping the temperature below 10°C. The mixture was allowed to come to room temperature overnight and then quenched with 6 N NaOH and enough water to dissolve the resulting solid. Phases were separated, and the aqueous phase was extracted with dichloromethane. The aqueous phase was cooled to 0°C and acidified with concentrated HCl. The resulting solid methanesulfonamide was filtered and dried under reduced pressure. The crude dried product was generally adequately pure for subsequent reactions.
The methanesulfonamide (3 mmol) in DMF (15 mL) was stirred and cooled to 0°C under a positive nitrogen atmosphere. Sodium hydride (95%, 152 mg, 6 mmol) was added and the mixture stirred for an additional 2 h. The freshly prepared chloroamine solution (5 mmol) was added and the mixture stirred at 0°C for 4 h and allowed to come to room temperature with stirring overnight. The mixture was then poured into ice-water (50 mL) and extracted with hexanes (2×50 mL), and the combined hexane extracts were consecutively washed with 1 M HCl, 3 M NaOH, water, and saturated sodium chloride. Concentration afforded the desired reduction products in good to excellent yields as described in Tables 1 and 2. All products are known compounds with spectra and physical constants identical to those reported in the literature.References:[1] March, J. Advanced Organic Chemistry, 4th ed.; Wiley and Sons: New York, 1992; pp 721-722.
[3] (see below)[11] Coleman, G. H.; Johnson, H. L. Inorg. Synth. 1939, 1, 59-62.
[12] Schmitz, V. E.: Schramm, S.; Flamme, W.; Bicker, U. Z. Anorg. Allg. Chem. 1973, 396, 178-184.
[14] Goehring, R. R. In Encyclopedia of Reagents for Organic Synthesis; Paquette, L., Ed.; Wiley: New York, 1995; pp 1052-1053.
____ ___ __ _Below follows the full text of Ref. #3:Convenient halodeamination and hydrodeamination of primary aminesGuziec, Frank S., Jr.; Wei, Dongchu,
Journal of Organic Chemistry 57, 3772-6 (1992)
 Abstract
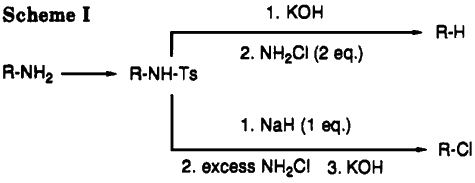
AbstractTreatment of p-toluenesulfonamides of primary amines with 2 equivalent of chloroamine at room temperature in the presence of base leads to reductive deamination. If excess chloroamine is present, the corresponding alkyl or aryl halides are obtained instead in good yields. Both reactions presumably proceed via tosylhydrazine and diazene intermediates; the course of the reaction is often governed by steric hindrance. Treatment of the isolated tosylhydrazine intermediate with base and chloroamine, bromine, or iodine also leads to the corresponding halides in very good yields. Thus, reaction of dehydroabietane
I (R = NHSO
2C
6H
4Me-p) with ClNH
2/Et
2O and KOH/MeOH gave 64%
I (R = H), whereas, with NaH/THF, NH
2Cl/Et
2O, and KOH/MeOH, 61%
I (R = Cl) was obtained.