Reduction with Sodium Borohydride-Transition Metal Salt Systems. I.1
Reduction of Aromatic Nitro Compounds with the Sodium Borohydride-Nickelous Chloride SystemAtsuko Nose and Tadahiro KudoChem. Pharm. Bull. 29(4), 1159-1161 (1981)
(
https://www.thevespiary.org/rhodium/Rhodium/pdf/nitrobenzene2aniline.nabh4-nicl2.pdf)
SummaryThe reduction of aromatic nitro compounds with the sodium borohydride-nickelous chloride system was examined. Aromatic nitro compounds afforded primary amines in high yield without by-products. Similarly, nitroso-, azoxy-, azo- and hydroxylaminobenzene were reduced with sodium borohydride-nickelous chloride to give aniline.
In recent years, significant advances have been made in the reduction of a variety of functional groups with sodium borohydride.
2 However, in general, sodium borohydride hardly reduces aromatic nitro compounds under ordinary conditions. In the previous paper,
3 it was reported that aromatic nitro compounds afforded the corresponding azoxy, azo and primary amine derivatives on heating directly with sodium borohydride.
Interestingly, it has recently been reported that aromatic nitro compounds can be reduced with sodium borohydride-transition metal salt systems, such as NaBH
4-CoCl
2,
4a,b NaBH
4-Co(pyridyl)
25 and NaBH
4-palladized charcoal.
6 In the present work, we investigated the sodium borohydride-nickelous chloride system in order to improve the yield in the reduction of aromatic nitro compounds with sodium borohydride-transition metal salt systems.
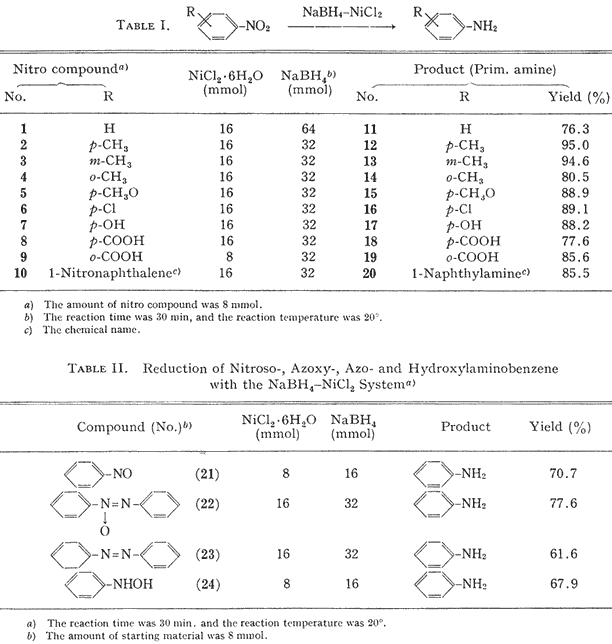
The reaction of aromatic nitro compounds with sodium borohydride-nickelous chloride hexahydrate at room temperature gave the corresponding primary amines in good yields. As shown in
Table I, irrespective of the existence of an electron-donating or electron-withdrawing substituent, these reduction proceeded to give the corresponding primary amines without byproducts. It appears that the sodium borohydride-nickelous chloride system is superior to the cobaltous chloride-sodium borohydride system
4a for the reduction of aromatic nitro compounds.
According to the method described above, nitrosobenzene (
21), azoxybenzene (
22), azobenzene (
23) and phenylhydroxylamine (
24) were reduced to aniline. It was reported that azoxybenzene and azobenzene were reduced to hydrazobenzene with sodium borohydride-cobaltous chloride hexahydrate.
7 Thus, it appears that the reducing power of the sodium borohydride-nickelous chloride system is greater than that of the sodium borohydride-cobaltous chloride system.
It can be presumed that the sodium borohydride-nickelous chloride system provides an useful and simple synthetic route under mild conditions for the preparation of aromatic primary amines from nitro compounds.
ExperimentalCommercially available nickelous chloride hexahydrate and sodium borohydride were used throughout this work.
The procedures for the reduction of
2 and
7 with sodium borohydride-nickelous chloride system will be described in detail as typical examples. The other aromatic nitro compounds,
21,
22,
23 and
24 were reduced similarly, and the reaction conditions are listed in
Tables I and
II. All spectral data of products were identical with those of the corresponding authentic samples.
Reduction of 2Compound
2 (1.10 g, 8 mmol) and nickelous chloride hexahydrate (3.80 g, 16 mmol) were dissolved in 99% methanol (30 ml) and sodium borohydride (1.21 g, 32 mmol) was added in portions with stirring under cooling for 30 minutes, then the stirring was continued for 30 minutes at room temperature (20°C). After the removal of methanol by distillation, the black precipitate was dissolved in 10% hydrochloric acid (
7,
8 and
9 were dissolved in conc. hydrochloric acid), then the acidic solution was basified by the addition of conc. ammonium hydroxide and extracted with ethyl acetate and the solution was dried over magnesium sulfate. After evaporating off the ethyl acetate, the residue was distilled under reduced pressure to give 813 mg (95.0%) of
12, bp 103-104°C (30 mmHg) (lit.
8 bp 200.35°C), mp 44-45°C (lit.
8 mp 43.5°C). All spectral data of
12 were identical with those of an authentic sample.
Reduction of 7Compound
7 (873 mg, 8 mmol) and nickelous chloride hexahydrate (3.80 g, 16 mmol) were dissolved in 99% methanol (30 ml) and sodium borohydride (1.21 g, 32 mmol) was added in portions with stirring under cooling for 30 minutes, then the stirring was continued for 30 minutes at room temperature (20°C). After usual work-up as described above, the residue was crystallized from water to give 770 mg (88.2%) of
17, colorless plates, mp 188-190°C (lit.
9) mp 189-190°C). This was identical with an authentic sample on the basis of mixed mp determination, and comparison of IR and UV spectra.
 References and Notes[1]
References and Notes[1] This work was presented at the 99th Annual Meeting of the Pharmaceutical Society of Japan, Aug. 1979, Sapporo, Abstract 30E 2-5.
[2] S. Yamada, Yuki Gosei Kagaku Kyokai Shi, 28, 1083 (1970)
[3] A. Nose and T. Kudo, Yakugaku Zasshi, 97, 116 (1977)
[4] a) T. Satoh and S. Suzuki, Tetrahedron Lett., 4555 (1969);
b) T. Satoh and S. Suzuki, Chem. Ind. (London), 1626 (1970)
[5] A.A. Vlcek and A. Rusina, Proc. Chem. Soc. (London), 161 (1961)
[6] T. Neilson, H.C.S. Wood, and A.G. Wylie, J. Chem. Soc., 371 (1962)
[7] A. Kasahara and T. Motomiya, Nippon Kagaku Zasshi, 86, 1343 (1965)
[8] J.F.T. Berliner and O.E. May, J. Am. Chem. Soc., 49, 1008 (1927)
[9] S.A. Dunn, J. Am. Chem. Soc., 76, 6191 (1954)