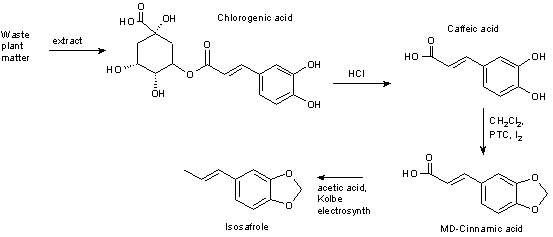
1) Isolate Chlorogenic acid
2) Hydrolysis to caffeic acid (requires HCl)
3) Convert caffeic acid to MD-cinnamic acid (requires DCM, a PTC and a trace of Iodine)
4) reduce the alkene bond
5) dihydro-MD-cinnamic acid + acetic acid gives MDP2P using the Kolbe electrosynth.
Comments please, especially on the Kolbe electrosynth. Has anyone tried this? Could I get away with lead, carbon or stainless steel electrodes? If I must have precious metal electrodes then can I use thinly plated electrodes?
Notes (from Merck XII ed):
1) Isolation of caffeic acid from green coffee,
Wolfrom et al, J. Agr.Food Chem.,
8, 58, (1960)
2) Isolation of caffeic acid from roasted coffee,
Krasemann. Arch. Pharm.
293, 721 (1960).
3) Hydroysis of Chlorogenic acid.
a) Fiedler, Arzneimittel-Forsch.
4, 41, (1954)
b) Whiting, Carr. Nature
180, 1479 (1957)
c) Guern. CA
61:9965h (1964)
4) Kolbe Electosynth.
a) B. C. L. Weedon, Quart. Rev.
6, 380 (1952)
b) A. K. Vijh, B. E. Conway, Chem. Rev.
67, 623 (1967)
c) H. J. Schaffer, Comp. Org. Syn.
3, 633-658, (1991)
d) L Eberson in "Organic Electrochemistry" ed Baizer (Dekker, NY 1973)