Novel diacid accelerated borane reducing agent for iminesZhi-Hui Lu, Nandkumar Bhongle, Xiping Su, Seth Ribe and Chris H. SenanayakeTetrahedron Letters 43, 8617–8620 (2002)
(
https://www.thevespiary.org/rhodium/Rhodium/pdf/grignardimine.amph-2002.pdf)
 Abstract
AbstractA remarkable effect of diacids in modulating the reactivity of borane has been discovered. This novel process provides a rapid and excellent access for reduction of a variety of imines with different functionalities.
____ ___ __ _
New resolution approach for large-scale preparation of enantiopure didesmethylsibutramine (DDMS)Zhengxu Han, Dhileepkumar Krishnamurthy, Q. Kevin Fang, Stephen A. Wald and Chris H. SenanayakeTetrahedron: Asymmetry 14, 3553–3556 (2003)
(
https://www.thevespiary.org/rhodium/Rhodium/pdf/grignardimine.amph-2003.pdf)
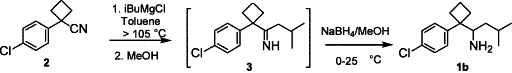 Abstract
AbstractAn improved synthesis and efficient resolution method to prepare both enantiopures of DDMS using crystallization of enantiomerically pure tartaric acid salts of racemic DDMS are disclosed.
Experimental
A 1 L three-necked round-bottomed flask was charged with 1-(4-chlorophenyl)-1-cyclobutylcarbonitrile (CCBC, 50.0 g, 261 mmol) and toluene (150 mL), followed by iso-butyl magnesium chloride (395 mL, 1.0 M in MTBE), and the resulting mixture was distilled until the internal temperature reached 105°C. After stirring at that temperature for 2 h, the mixture was cooled to 0°C and methanol was added slowly (295 mL), followed by sodium borohydride (10.4 g, 1.06 equiv.) portion-wise. The resulting mixture was stirred at rt for 15 min and was added to a 2N HCl solution (330 mL) slowly, stirred for 15 min and the phases were separated. The aqueous phase was extracted with toluene (300 mL), the combined organic phases were distilled to remove methanol, and then washed with aqueous NaOH solution (1.5 M, 100 mL) and water (100 mL) twice. The resulting organic phase was heated to 50–60°C, followed by an addition of D-tartaric acid (40.0 g) in water (80 mL) and acetone (40 mL) slowly. The reaction mixture was azeotrope distilled until the internal temperature reached 92°C and then cooled to ambient temperature in 1–2 h. The slurry was filtered, and the wet cake was washed with MTBE (100 mL) and dried at 40–45°C under reduced pressure to afford (RS)-DDMS·D-TA (100.5 g) in 95.8% yield.____ ___ __ _
A Study and Identification of Potential By-Products of SibutramineG. Om Reddy, M. R. Sarma, B. Chandrasekhar, J. Moses Babu, A. S. R. Prasad, and C. M. Haricharan RajuOrganic Process Research & Development 3, 488-492 (1999)
(
https://www.thevespiary.org/rhodium/Rhodium/pdf/grignardimine.byproducts.pdf)
AbstractIn the synthesis and process development of sibutramine (
9), the isolation and characterization of two potential by-products namely heptane dinitriles (
4a-b) and bis-cyclobutyl alkylamine (
10) have been studied. The key steps in the synthesis of sibutramine which have contributed to the formation of above by-products are cycloalkylation of 4-chlorophenyl acetonitrile (
1) and tandem Grignard reduction on 1-(4-chlorophenyl)-cyclobutyl carbonitrile (
3).
____ ___ __ _
Synthesis of sibutramine, a novel cyclobutylalkylamine useful in the treatment of obesity, and its major human metabolitesJ.E. Jeffery, F.Kerrigan, T.K. Miller, G.J. Smith and G.B. Tometzki,
J. Chem. Soc. Perkin Trans. 1, 2583-2589 (1996)DOI:
10.1039/P19960002583
AbstractSynthetic routes to N-{1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutyl}-N,N-dimethylamine (sibutramine)
1 and its demethylated and hydroxylated human metabolites N-{1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutyl}-N-methylamine
2, 1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutylamine
3, 4-amino-4-[1-(4-chlorophenyl)cyclobutyl]-2-methylbutan-1-ol
4 and c-3-(1-amino-3-methylbutyl)-3-(4-chlorophenyl)cyclobutan-r-1-ol
5a are described. Key steps are tandem Grignard–reduction reactions on 1-(4-chlorophenyl)cyclobutanecarbonitrile
7 and its 3-(tetrahydropyran-2-yloxy)-substituted analogue
14 and a convenient one-pot conversion of 4-chlorophenylacetonitrile
6 into the 3-hydroxycyclobutanecarbonitrile
13.