The synthesis of phenylpropanoids makes part of the shikimic acid pathway. Phenylpropanoids with 4-OH, 4-OMe, 3-MeO-4-OH, 3,4-MeOH, 3,4-OH are very common. Methylenedioxyrings are less common but usually can still be found in a majority of plants. However, not all substances bearing methylenedioxyrings can be used as piperonal/(iso)safrole precursor, since methylenedioxy-bearing compounds are often applied as (neo)lignane, alkaloid, flavanoid, ... construction block. Go to a library and look for books on plant biosynthesis and the like. Going through these books for a handful of hours is usually enough to understand it is not as easy as 1-2-3...

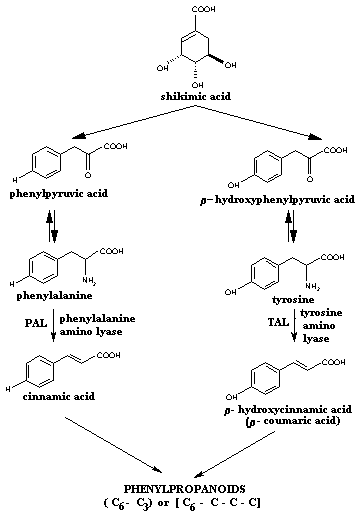
Shikimic acid pathway:
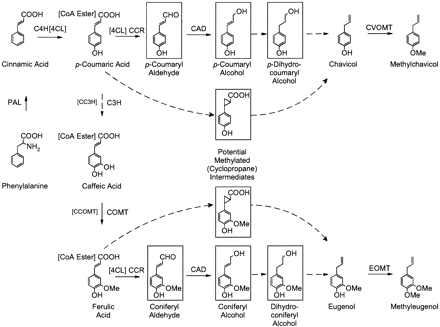
Biosynthesis of methylchavicol/eugenol in sweet basil:
3,5-diMeO-4-OH is also very common...
