No, but I knew that his references for that particular reaction was good. Here it follows:The Preparation of Aliphatic Amidesby JA Mitchell and EE Reid
J Am Chem Soc 53, 1879-1883 (1931)The most satisfactory methods of preparing amides have involved dehydration of the ammonium salt of the corresponding acid. Hofmann [1] prepared various amides by heating ammonium salts of aliphatic acids for five or six hours at 230°C under pressure. Kundig [2] prepared acetamide by the rapid distillation of ammonium acetate and by heating an alcoholic solution of acetic acid and ammonia in a sealed tube for a long time at 100°C. He also obtained a yield of amide greater than 25% by passing dry ammonia through acetic acid and then heating to boiling. Grant and James [3] have prepared amides by saturating the acid with dry ammonia and boiling. Dunlap, [4] Keller [5] and Verley [6] have modified the procedure by heating sodium acetate and ammonium chloride at 240°C.
Acetamide is best prepared according to Coleman and Alvarado [7] by heating a mixture of ammonium acetate and acetic acid under a reflux condenser in such a manner that the excess acetic acid and the water formed in the reaction are slowly distilled off [8]. Noyes and Goebel have also shown that acetic acid catalyzes the reaction.
This method is very satisfactory for preparing acetamide, but is not economical for the higher aliphatic acids on account of the cost of the large excess of acid required. This suggested using an excess of the other reactant, ammonia, which is cheap. Instances in which dimethyl amides were prepared in a similar manner are rare. Franchimont and Klobbie [9] prepared dimethyl heptoamide by heating the acid with dimethylamine at 230°C in a sealed tube. Verley [6] prepared dimethyl formamide and acetamide by distilling a mixture of an alkali salt of the acid and dimethylamine hydrochloride.
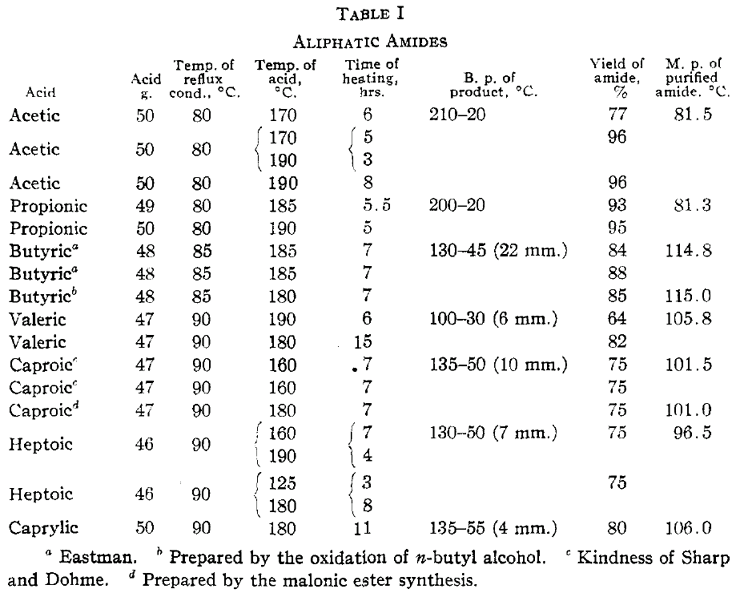
ResultsWe have found that by passing ammonia gas through aliphatic acids kept at a suitable temperature in such a way that the water formed is continually removed, the equilibrium point of the reaction may be displaced far to the amide side and very high yields of amides obtained. The results with the aliphatic amides are indicated in Table I.
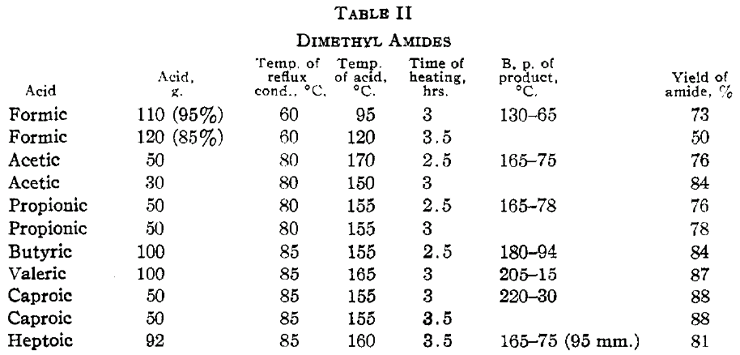
The temperature at which the acid is heated is sufficient to dehydrate a small proportion of the higher amides to form the nitriles. This reaction becomes appreciable with butyric and the higher amides. The reaction velocity is appreciably slower with the higher acids. Heptoic acid reacts much more slowly than caproic and caprylic more slowly than heptoic. Lauric acid was heated at 185°C for sixteen hours. Only a small fraction was converted to the amide. No amide was obtained from palmitic or stearic acid upon heating either at 125 or 190°C for considerable intervals of time. The ammonium salts of these acids are probably unstable under the conditions of the experiments. Similar experiments were performed in which anhydrous zinc chloride was added to acetic, propionic and caproic acids. No difference in rate of formation of the amide was observed when compared with parallel experiments with no zinc chloride. Zinc chloride is a catalyst, however, for dehydration of the amide to the nitrile. At a temperature higher than that used in the preparation of the amide a considerable proportion of caproic acid is converted to the nitrile. Although small amounts of nitrile are formed in distilling acetamide and propionamide, they are satisfactorily purified by distillation at atmospheric pressure. It is better to fractionate the higher amides at reduced pressures. Acetamide is best recrystallized from methyl alcohol and ether according to the method outlined by Wagner [10]. The amides become increasingly soluble in ether as the molecular weight increases. This furnishes a satisfactory recrystallization medium for all but acetamide. The amides are more effectively recrystallized from hot benzene. The crystals, however, tenaciously absorb the solvent and it is advisable to centrifuge. Dimethyl amides were prepared in the same manner, the ammonia being replaced by dimethylamine. The results are given in Table II. The dimethyl amides obtained were all liquids at ordinary temperatures and were purified by distillation.
 Experimental
ExperimentalThe apparatus employed. consisted of a flask with a jacketed reflux condenser. The condenser was connected top and bottom with a side tube of the same length, which upon being filled with water could be kept at any desired temperature up to 90°C by means of a Bunsen burner placed at the lower end of the side-tube. The flask containing the acid was heated to any desired temperature by an oil-bath. Temperatures were read from a thermometer which was placed inside the flask and extended into the condenser. The ammonia delivery tube entered at the top of the condenser and extended to the bottom of the flask.
For the preparation of the amides a stream of ammonia, taken directly from a cylinder, was passed through the delivery tube and bubbled through the acid the entire duration of the experiment. The water formed in the reaction was swept out through a tube just at the top of the condenser jacket into a bulb.
Samples of amides were carefully purified. After the preliminary vacuum distillation the amides were further purified by crystallization from pure benzene and absolute ether, frequently centrifuging and drying over sulfuric acid in a vacuum desiccator until accurate melting points were obtained, checking for several recrystallizations. The melting points were taken with a calibrated Anschütz thermometer. Every precaution was taken to prevent overheating of the melting point bath and its temperature was kept uniform by efficient stirring. Melting points quoted in Table I are the corrected ones.
Acetamide was purified by recrystallizing four times from methyl alcohol and ether, centrifuging twice. Propionamide was crystallized once from methyl alcohol and ether, followed by centrifuging, and then recrystallized twice from ether. Butyramide (a) was crystallized from benzene, centrifuged and recrystallized three times from ether, followed by centrifuging. Butyramide (b) was crystallized three times from ether, and centrifuged. Valeramide was crystallized three times from ether and centrifuged. Caproamide (c) was crystallized three times from ether and centrifuged twice. Caproamide, (d) heptoamide and caprylamide were crystallized from benzene, centrifuged, and recrystallized twice from ether.
For the preparation of the dimethyl amides, the apparatus was attached to a water pump at the exit side and a current of air mixed with dimethylamine, prepared by dropping an aqueous solution of the amine [11] on solid potassium carbonate, was drawn through the acid during the course of the experiment. The gas was dried by passing it over solid potassium hydroxide. Certain experiments indicated, however, that drying was not essential. The reaction proceeds much faster with dimethylamine than with ammonia.
SummaryA satisfactory and economical method has been described for the prepara- tion of amides and dimethyl amides from the normal acids of the aliphatic series up to caprylic. Accurate melting points of the amides from acetic to caprylic have been determined.
References1) Hofmann, Ber., 15, 977 (1882).
2) Kundig, Ann., 105, 277 (1858).
3) Grant and James, J. Am. Chem. Soc., 39, 933 (1917).
4) Dunlap, ibid., 24, 762 (1902).
5) Keller, J. prakt. Chem., [2] 31, 364 (1885).
6) Verley, Bull. soc. chim., [3] 9, 691 (1893).
7) Coleman and Alvarado, "Organic Syntheses," 1923, Vol. 3, p. 2.

Rosanoff, Gulick and Larkin, J. Am. Chem. Soc., 33, 974 (1911);
Hitch and Gilbert, ibid., 35, 1780 (1913);
Noyes and Goebel, ibid., 44, 2286 (1922).
9) Franchimont and Klobbie, Rec. trav. chim., 6, 247 (1887).
10) Wagner, J. Chem. Ed., 7, 1135 (1930).
Wagner obtained a melting point as high as 83°C for acetamide. We obtained a melting point of only 81.5°C after a very rigid purification.