Hi! The topic of today is the total synthesis of 5-MeO-DMT, which despite having seven steps STILL gives 50% yield. And the procedure still has much room for improvements!
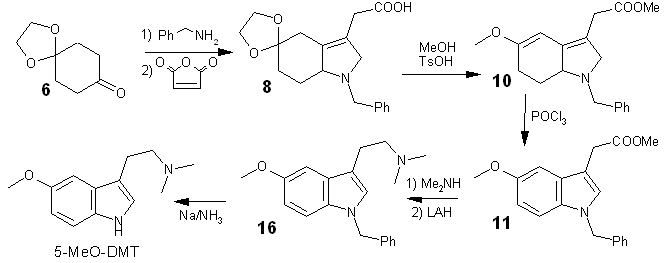
N-(1,4-Dioxaspiro[4.5]decyl-7-idene)benzylamine (7). A solution of 10.1 g (65.0 mmol) of commercial 1,4-cyclohexanedione mono-ethylene ketal 6 and 8.00 mL (73.3 mmol) of benzylamine in 50 mL of toluene was heated under reflux in a Dean-Stark apparatus for 6 h. The solvent was removed under reduced pressure affording the crude imine 7 which was directly used for the next step.
1'-Benzyl-2'-oxo-1',2',4',6',7',7a'-hexahydrospiro[1,3-dioxolane-2,5'-[5H]indole]-3'-acetic Acid ( .
.A solution of (7.32 g, 74.7 mmol, 1.2 equiv) of maleic anhydride in 20 mL of THF was added dropwise to a solution of the crude imine 7 above, in 20 mL of THF at 0°C. After stirring for 1 h, the crystalline adduct 8 was filtered. Evaporation of the solvent gave a residue which was purified by FC (80:20, then 100:0) affording additional adduct 8. An analytical sample was obtained by recrystallization: mp 199-201°C (EtOAc/MeOH).
Methyl 1-Benzyl-5-methoxy-2-oxo-2,6,7,7a-tetrahydro-1H-indole-3-acetate (10)The crude acid 8 from the above step, 200 mL of methanol, 20 mL (3 equiv) of 2,2-dimethoxypropane, and 3.00g of PTSA was heated at reflux temperature 4h. After evaporation of the solvent, the residue was diluted with EtOAc and washed with a saturated solution of NaHCO3. A FC (80:20) afforded 80 mg of oxindole 10a (vide infra) and 19.3 g (91% yield from ketone 6) of viscous ester 10.
Methyl 1-Benzyl-5-methoxy-1H-indole-3-acetate (11)To a solution of 6.50 g (19.9 mmol) of ester 10 in 22 mL of acetonitrile and 5.3 mL (65.7 mmol, 3 equiv) of pyridine was added dropwise 4.10 mL (44.0 mmol, 2 equiv) of POCl3, and the mixture was stirred at 60 C for 1 h. After cooling, water was added, and the mixture was extracted with ether. The organic layer was washed with a saturated solution of NaHCO3, followed by the usual workup. A FC (20:80) afforded 5.03 g of oily indole 11 in 82% yield (75% from ketone 6).
1-Benzyl-5-methoxy-N,N-dimethyl-1H-indole-3-acetamide (15)In a closed vial, a solution of 2.40 g (7.80 mmol) of ester 11 and 19 mg of NaCN in 30 mL of a Me2NH (10 M) solution in methanol was heated at 50 C for 4 days. After evaporation of the amine excess and the solvent under reduced pressure, addition of ether brought about the crystallization of amide 15 which was filtered, washed with water and ether, and dried over P2O5 (2.50 g, 90% yield). The same reaction carried out with 8.86 g (29.0 mmol) of ester 11 in 76 mL of the Me2NH solution, at room temperature for 6 days, afforded 9.40 g (91% yield) of amide 15. An analytical sample was obtained by recrystallization: mp 128 C (EtOAc).
2-(1-Benzyl-5-methoxy-1H-indol-3-yl)-N,N-dimethylethylamine (16)To a suspension of 1.41 g (37.2 mmol) of LiAlH4 in 300 mL of ether was added 4.00 g (12.4 mmol) of amide 15, and the mixture was heated at reflux temperature for 48 h. After cooling, 5 mL of a 20% solution of potassium sodium tartrate was added dropwise. The aluminum complexes were filtered on Celite and after evaporation of the filtrate, a FC (EtOH/EtOAc 50:50 + 5% NH4OH) afforded 3.40 g (90% yield) of oily amine 16.
N,N-dimethyl-5-Methoxy-Tryptamine (5-MeO-DMT)In 15 mL of THF was condensed at -78°C ca. 100 mL NH3, followed by the addition of 1.57 g (68.4 mmol, 6 equiv) of Na (blue color). A solution of 3.51 g (11.4 mmol) of indole 16 in 15 mL of THF was added dropwise, and the mixture was further stirred at -33°C for 1 h 30. Isoprene was next added dropwise at -50°C until total discoloration of the mixture occurred (2.35mL), followed by the addition of 2 g of NH4Cl. After NH3 and solvent evaporation, water was added, and the mixture was extracted with ether (K2CO3 drying). A FC (MeOH/EtOAc 50/50 + 5% NH4OH) afforded 2.00 g (80% yield) of crystalline 5-MeO-DMT. An analytical sample was obtained by recrystallization: mp 68°C (cyclohexane).