I have never before seen such a well-hidden synthesis of methamphetamine in an article before - it is really hard to spot that they are actually synthesizing it from one of their isoxazolines (check the article yourself).2-Methylisoxazolin-5-ones. Part I.F. DeSarlo, L. Fabbrini, G. RenziTetrahedron 22, 2989-2994 (1966)
(
https://www.thevespiary.org/rhodium/Rhodium/pdf/meth.2-methylisoxazolin-5-one.pdf)
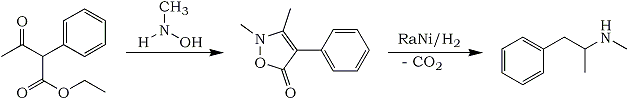 2,3-Dimethyl-4-phenylisoxazolin-5-one (IX)
2,3-Dimethyl-4-phenylisoxazolin-5-one (IX) Ethyl
alpha-phenylacetoacetate (0.1 mole) and N-methylhydroxylamine hydrochloride (0.45 mole) in anhydrous pyridine (100 ml) were heated at 100°C for 12 hr. The residue after evaporation of the solvent was washed with ether, then with water, yield 91%; mp 112-113°C (ligroin). In alcohol, as reaction solvent, the yield was 73%.
Hydrogenation of IXA solution of
IX (0.02 moles) in 150 ml anhydrous EtOH
* was shaken for 1 day under a 3-4 atm. H
2 pressure, with Raney Ni. After the absorption of 0.04 moles H
2, the solution was filtered and fractionated to isolate N-Methylamphetamine (
XIV); bp 38°C/0.3 torr; 208°C/750 torr. mp of the hydrochloride 129-134°C.
* If 95% EtOH was used instead of anhydrous, the compound only absorbed 1 equivalent of H
2 and the product was instead the intermediate imine.
Precursor SynthesisEthyl Phenylacetate + Ethyl Acetate -> Ethyl alpha-Phenylacetoacetate J. Amer. Chem. Soc. 69, 119 (1947)
J. Chem. Soc. 123, 1764 (1923)
Chem. Ber. 63, 1557 (1930)
Chem. Ber. 66, 1512 (1933)
Phenylacetonitrile + Ethyl Acetate -> PhenylacetoacetonitrileOrganic Syntheses, Coll. Vol. 2, p. 487
(
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv2p0487)
Phenylacetoacetonitrile -> Ethyl alpha-PhenylacetoacetateOrganic Syntheses, Coll. Vol. 2, p. 284
(
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv2p0284)