I had a look, and there are no literature references available about the pharmacological effects of Phenylalaninol Methyl Ether (amphetamine with a methoxy group on its alpha-methyl group). However, below follows a synthesis of it:
Synthesis of alpha-methoxymethyl-phenethylamine (Phenylalaninol methyl ether)J. Org. Chern., 43(5), 892-898 (1978)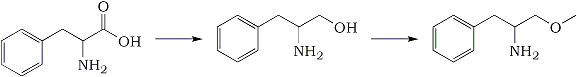 Phenylalanine -> Phenylalaninol -> Phenylalaninol Methyl Ether(S)-(-)-Phenylalaninol
Phenylalanine -> Phenylalaninol -> Phenylalaninol Methyl Ether(S)-(-)-PhenylalaninolThis was prepared according to the method of Yamada
[14] mp 88-90°C, [a]25D -25.4° (c 1.22, EtOH). Alternatively, it was prepared by the procedure of Brown's
[15] and that of Lane
[16] and all three methods gave similar results. The latter method
[16], in our hands, proved to be the most convenient.
(S)-(-)-2-Amino-1-methoxy-3-phenylpropaneA solution of 18.4 g (0.122 mol) of (R)-(-)-phenylalaninol in 250 mL of anhydrous tetrahydrofuran was added dropwise to a stirred suspension of 5.23 g (0.130 mol) of potassium hydride (pentane washed) in 100 mL of tetrahydrofuran at 25°C under nitrogen. The resulting pale yellow gelatinous mixture was allowed to stand overnight and then a solution of 17.0g (0.119 mol) of methyl iodide in 150 mL of THF was added dropwise over 2 h. Mixing was accomplished by external shaking, since the gelatinous mixture would not stir with magnetic stirring bars. The reaction components were allowed to mix an additional 3 hand then poured into 1 L of cold saturated brine, extracted with ether (3x), dried with Na
2SO
4, and concentrated to give 24.9g of crude product. Distillation gave 17.1g (bp 55-59°C/0.1mmHg) of a clear oil which on standing became cloudy and rapidly produced a white precipitate which was found to be the carbonate. It was subsequently found that conversion of the freshly distilled methoxyamine to its hydrochloride salt was a more convenient way to store the compound. Thus the methoxyamine (17.0g), immediately after distillation, was dissolved in 700 mL of absolute ethanol and dry HCl bubbled in for 10 min. The resulting solution was concentrated, in vacuo, to furnish 20.5g of a colorless solid which was recrystallized from ethanol-ether (13:1), mp 151-152°C.
To release the free methoxyamine, it was dissolved in 5% potassium carbonate solution and extracted with ether, dried (Na
2SO
4), and concentrated. Bulb-to-bulb distillation (bp 52°C/0.1mmHg) gave the product as a clear oil.
References[14] H. Seki, K. Koga, H. Matsuo. S. Ohki, I. Matsuo, and S. Yamada, Chem. Pharm. Bull., 13, 995 (1965).
[15] N. M. Yoon, C. S. Pak. H. C. Brown, S. Krishnamurthy, and T. P. Stocky, J. Org. Chem., 38, 2786 (1973).
[16] C. F. Lane (Aldrich-Boranes, inc.),
Patent US3935280