While it is true that this rxn is no good for safrole, the reason for that has absolutely nothing to do with xcess sulfuric acid.
In fact, there's no need for high conc of H2SO4 in this rxn (have nice proc's, can post if interested) - its sole role is to create a carbanion from the alkene, in our case this is methylenedioxyphenyl-isopropyl (with "+" being situated on the middle atom of the chain). This carbanion then reacts w/acetonitrile which then rearranges to acetamide.
The substrates that can bee used in Ritter rxn need not bee specifically alkenes - any cpd that forms stable carbanions will do (anions must bee stable since nitrile group is a relatively poor 'trap' for them).
Now here's the real pitfall
(from now on the ideas and drawings beelong to Fomalhaut of HyperLab, from Post 404131 (missing)
(Fomalhaut: "Íåìíîãî òåîðèè...", Russian HyperLab)):

The positive charge on the nitrogen in nitrillium anion migrates to the adjacent carbon, which, being a perfect electrophil, plunges into the nearby ring, forming that methylenedioxy-dihydroisoquinoline.
Here's a very similar procedure from Titze & Eicher: dihydroisoquinoline synth:
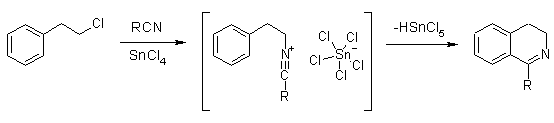
As you can see, they use a FC catalyst here.
But, the MAIN reason that Graaf-Ritter doesn't work on safrole is the fact that methylendioxyring is highly activated and thus is much much more prone to cyclization

But, OTOH, that offers us some other possibilities. You could Graaf-Ritter 'straight' allylbenzene and do next to anything to it (in terms of ring substitution, i mean), while it's still in acetamide-protected form.
Antoncho