Synthesis of the Yeast Antioxidant Benzofuran and AnaloguesBrian A. McKittrick and Robert StevensonJ. Chem. Soc. Perkin Trans. I, 709-712 (1984)
(
https://www.thevespiary.org/rhodium/Rhodium/pdf/croweacinaldehyde.pdf)
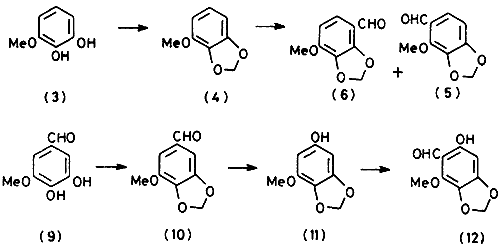
Methylenation of 3-methoxypyrocatechol (
3) by treatment with methylene dibromide and potassium carbonate in dimethyl sulphoxide afforded 1-methoxy-2,3-methylenedioxybenzene (
4) in about 80% yield. Several reports concerning the Vilsmeier formylation of (
4) exist. In the first. Brownell and Weston
9 reported the isolation of 2-methoxy-3,4-methylenedioxybenzaldehyde (
5) as the sole product, a conclusion supported by later findings
10. In contrast, Wagner and co-workers
3,11 found that the product was a mixture of the aldehyde (
5) and 4-methoxy-2,3-methylenedioxybenzaldehyde (
6). This was substantiated by later work of Shulgin
12 and Dallacker
13, although there was a wide variation in the ratios of the two aldehydes reported. In our hands, the crude product obtained from compound (
4) by treatment with phosphorus oxychloride and dimethylformamide furnished the isomers (
5) and (
6) in a 2:1 ratio, as estimated from integration of the
1H NMR spectrum. The mixture was separated by chromatography on silica gel.
The readily available 3,4-dihydroxy-5-methoxybenzaldehyde (
9)
16 was converted into the methylenedioxy derivative (
10) by an improved procedure and then into the phenol (
11) by Baeyer-Villiger oxidation with performic acid. Gattermann formylation of (
11) with zinc cyanide gratifyingly yielded the required aldehyde (
12) in over 80% yield.
 Experimental
ExperimentalMp's were determined with a Gallenkamp apparatus and are uncorrected. The silica gel used for chromatography was J. T. Baker (40-140 mesh) and light petroleum refers to the fraction boiling in the range 54-105°C. Ether refers to diethyl ether.
1-Methoxy-2,3-methylenedioxybenzene (4)Anhydrous potassium carbonate (20 g) followed by dibromomethane (10 ml) were added to a solution of 3-methoxypyrocatechol (
3) (11.3 g) in dimethyl sulphoxide (250 ml). The mixture was heated at 85°C for 2 h under nitrogen, then cooled and poured into water (250 ml). Extraction with ether (5 x 100 ml) and evaporation of the washed and dried (MgSO
4) extract yielded an oil, which was distilled (65-75°C bath temperature at 0.05 mmHg). On standing, the distillate gave compound (
4) as light yellow crystals (9.67 g), mp 39-41°C (lit.
19 mp 41°C).
2-Methoxy-3,4-methylenedioxybenzaldehyde (5) Phosphorus oxychloride (9.0 ml) was added dropwise during 10 min at room temperature to a solution of compound (
4) (5.44 g) in dimethylformamide (7 ml). The mixture was stirred at room temperature for 30 min, then at 50°C for 1 h and at 90°C for 2 h, cooled, and diluted with water (50 ml) to yield a yellow precipitate which was dissolved in ether, washed with water, and dried. Evaporation of this extract gave a solid (4.5 g) whose integrated
1H NMR spectrum indicated the presence of isomers (
5) and (
6) in a 2:1 ratio. Chromatography of this mixture on silica gel with light petroleum–dichloromethane (3:7) yielded first the aldehyde (
5) as needles (2.48 g) from ethanol, mp 102-104°C (lit.
3 mp 103-105°C).
Continued elution with the same eluant gave mixtures of isomers (
5) and (
6), followed by pure 4-methoxy-2,3-methylenedioxybenzaldehyde (
6), obtained as a solid (1.3 g), mp 82-85°C (lit.
3 85-86°C).
3-Methoxy-4,5-methylenedioxybenzaldehyde (10)Anhydrous potassium carbonate (20 g) and dibromomethane (10 ml) were added to a solution of 3,4-dihydroxy-5-methoxy-benzaldehyde (
9) (13.5 g) in dimethyl sulphoxide (250 ml) and the mixture was heated at 90°C for 2.5 h under nitrogen. Dilution of the cooled mixture with water was followed by chloroform extraction (600 ml). Evaporation of the washed and dried extract gave a residual solid which on distillation (110-115°C at 0.5 mmHg) gave the methoxymethylenedioxy aldehyde (
10), mp 130-132°C (lit.
16 mp 131-132°C).
3-Methoxy-4,5-methylenedioxyphenol (11)Formic acid (90%; 40 ml) was added to hydrogen peroxide solution (30%; 100 ml) and stirred at 0-5°C for 1 h. A solution of the benzaldehyde (
10) (4.8 g) in formic acid (90%; 100 ml) was then added with continued stirring at 5°C for 3.5 h, after which additional performic acid (from 5 ml hydrogen peroxide and 20 ml formic acid) was then introduced. After the mixture had been stirred for a further 75 min, the reaction was quenched by the addition of sodium sulphite (10 g) and ice (400 ml) and extracted with ether (600 ml). After being washed with water, the extract was stirred vigorously at room temperature for 30 min with dilute sodium hydroxide solution (pH 12). The dark red basic phase was then separated, acidified with 3% hydrochloric acid, and re-extracted with ether. Evaporation of the washed and dried extract yielded a semi-solid residue which on distillation (ca. 100°C at 0.1 mmHg) gave the phenol (
11) as a light tan solid (2.02 g), mp 85-88°C (lit.
3 mp 89-91°C).
6-Hydroxy-2-methoxy-3,4-methylenedioxybenzaldehyde (12) Zinc cyanide (310 mg) was added to a solution of the phenol (
11) (180 mg) in ether (35 ml) and hydrogen chloride bubbled through the stirred mixture for 1.5 h at 0°C. Stirring was continued for a further 1.5 h after which the ether was decanted. Water (40 ml) was added to the gummy residue, and the mixture heated on the steam-bath for 15 min, then cooled and filtered. Recrystallization of the solid (175 mg) from light petroleum gave the aldehyde (
12) as cream flakes, mp 124-125°C.
References[3] J. Am. Chem. Soc. 81, 4983 (1959)
(
https://www.thevespiary.org/rhodium/Rhodium/pdf/methoxymethylenedioxybenzene.pdf)
[9] W. B. Brownell and A. W. Weston, J. Am. Chem. Soc., 73, 4971 (1951)
Post 484720
(Rhodium: "Croweacinaldehyde", Novel Discourse)[10] F. Benington and R. D. Morin, J. Org. Chem., 27, 142 (1962)
[11] A. F. Wagner and F. A. Kuehl, Jr.,
Patent US3000902
(Chem. Abstr., 1972, 56, 1339c).
[12] A. T. Shulgin, Can J. Chem., 46, 75 (1968)
Post 489559
(Rhodium: "Synthesis of Myristicinaldehyde/Apiolealdehyde", Novel Discourse)[13] F. Dallacker, Chem. Ber., 102, 2663 (1969)
Post 477701
(Rhodium: "Synthesis of all Apiole Positional Isomers", Novel Discourse)[16] G. E. Schneiders and R. Stevenson, J. Org. Chem., 46, 2969 (1981)
Post 477377
(Rhodium: "Myristicin-derived Cathinones (Precursor prep)", Novel Discourse)[19] K. N. Campbell, P. F. Hopper, and B. K. Campbell, J. Org. Chem., 16, 1736 (1951)
Post 489659
(Rhodium: "Myristicin & Myristicinaldehyde", Novel Discourse)