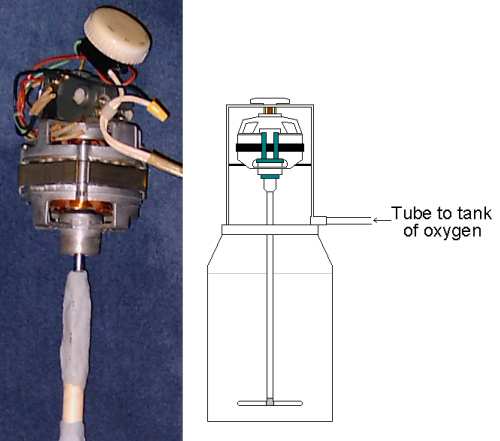
What's up?! My filthy cock-sucking internet provider was down for several hours, and it's great to be back online! Alright, lets say that you have a vessel with all the Wacker chems in it, which is under a bit of pressure with oxygen, but you don't want to make your arms soar by shaking the hell out of it, so you decide to use an electric stirrer. In order to keep the vessel under pressure, the stirrer must be enclosed within the same area as the vessel. Sure, I could try some bull-shit like keeping the engine outside the vessel and putting tight rubber gaskets around the stirring rod, so air won't escape, but that's unrealistic, because the stirring rod's home-made, so it's a little off-center. Anyway, if the engine's enclosed within a vessel of pure oxygen, would the oxygen ignite and blow my face off?
If that's the case, then here's another idea: Leave the vessel open with the engine stirring away, and have several tubes near the bottom of the solution bubbling in pure oxygen at a rapid rate. Would that insure intimate contact of oxygen with the solution?

Love my country, fear my government.