Their examples does not include nitro-alkenes, but all alkenes with any electron-withdrawing group whatsoever worked in their synthesis, so it is highly likely that this can be used to produce 1-(3-indolyl)-2-nitropropenes in a single step from indoles and 3-acetoxy-2-nitro-propene. This can in turn is made by condensing formaldehyde with nitromethane (yielding 2-nitro-propane-1,3-diol), acetylating the diol with Ac2O or AcCl and distilling in vacuo to give the nitroalkene (se refs at the bottom).Palladium-catalyzed functionalization of indoles with 2-acetoxymethyl substituted electron-deficient alkenesShengming Ma, and Shichao Yu,
Tet. Lett. 45(45), 8419-8422 (2004), DOI:
10.1016/j.tetlet.2004.08.178

AbstractA new functionalization of indoles via palladium-catalyzed reaction of indoles and 2-acetoxymethyl substituted electron-deficient alkenes is reported. The reaction was carried out under neutral condition and no isomerization of the carbon–carbon double bond was observed.
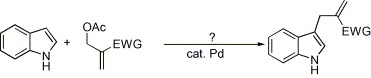 Preparation of 3-acetoxy-2-nitro-propene:Nitromethane + Formaldehyde
Preparation of 3-acetoxy-2-nitro-propene:Nitromethane + Formaldehyde --1:NaOH--2:Acetylation-->
3-acetoxy-2-nitro-propeneM.B. Frankel,
Tetrahedron Suppl. 4, 213-217 (1963)2-nitro-propane-1,3-diol + acetyl chloride --CH
2Cl
2-->
1,3-diacetoxy-2-nitro-propane --180°C/70-100mmHg-->
3-acetoxy-2-nitro-propeneKlager,K.;
Monatsh. Chem. 96, 1-8 (1965)2-nitro-propane-1,3-diol + acetic acid anhydride -> 1,3-diacetoxy-2-nitro-propaneCarbohydr. Res. 310(3) 191-202 (1998)