Friedel-Crafts Acylation with N-(Trifluoroacetyl)-?-amino Acid Chlorides. Application to the Preparation of ?-Arylalkylamines and 3-Substituted 1,2,3,4-TetrahydroisoquinolinesJ. E. Nordlander, M. J. Payne, F. G. Njoroge, M. A. Balk, G. D. Laikos, and V. M. Vishwanath
J. Org. Chem.,
49(22), 4107-4111.

(Ref. #13 from https://www.thevespiary.org/rhodium/Rhodium/pdf/nichols/nichols-dragonfly-2.pdf
.)Abstract Several
N-(trifluoroacetyl)-?-amino acid chlorides have been reacted with PhH, MeOPh, and 1,2-(MeO)
2PhH in the presence of AlCl
3 or SnCl
4 to produce ArCOCH(NHCOCF
3)R in fair to high yields. The (trifluoroacetyl)amino group of the products can be
N-methylated with MeI/K
2CO
3 in boiling Me
2CO. Reduction of the ketones with H
2 - Pd/C in EtOH under neutral conditions resulted in alcohols. Hydrogenation in the presence of HCl led to complete deoxygenation. Reductions with Et
3SiH in refluxing CF
3COOH or in BF
3·Et
2O at rt gave alcohols (Ar = Ph) or deoxygenated products (Ar = 4-MeOPh or 3,4-(MeO)
2Ph). The products of reduction can be readily detrifluoroacetylated by mild basic hydrolysis and thence converted to the corresponding 3-substituted 1,2,3,4-tetrahydroisoquinolines by condensation with CH
2O.
N-(Trifluoroacetyl)-?-amino Acid Chlorides as Chiral Reagents for Friedel-Crafts SynthesisJ. E. Nordlander, F. G. Njoroge, M. J. Payne, and D. Warman
J. Org. Chem.,
50, 3481-3484 (1985).

Abstract Chiral
N-(trifluoroacetyl)-?-amino acid chlorides undergo Friedel-Crafts reaction with PhH and 1,2-(MeO)
2PhH under mild conditions commonly with complete (>99%) preservation of configurational identity. The resultant (trifluoroacetyl)amino ketones may be deoxygenated with Et
3SiH or H
2/Pd-C in acidic media to the corresponding
N-(trifluoroacetyl)-?-arylalkylamines likewise without loss of configurational purity.
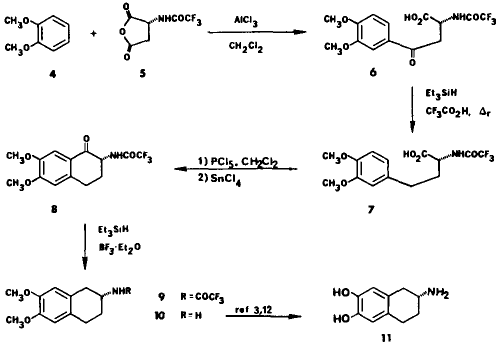
A Short Enantiospecific synthesis of 2-Amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene (ADTN)J. E. Nordlander, M. J. Payne, F. G. Njoroge, V. M. Vishwanath, Gi Rin Han, G. D. Laikos, and M. A. Balk
J. Org. Chem.,
50, 3619-3622 (1985).

A Short Synthesis of (S)-(+)-TylophorineJ. E. Nordlander† and F. G. Njoroge
J. Org. Chem.,
52, 1627-1630 (1987).

Abstract Friedel-Crafts acylation of 2,3,6,7-tetramethoxyphenanthrene with (
S)-
N-(trifluoroacetyl)prolyl chloride (1.2 eq.) followed by deoxygenation of the resultant ketone with Et
3SiH in BF
3·Et
2O at rt, removal of the trifluoroacetyl group (NH
3 - MeOH), and Pictet-Spengler cyclomethylenation with CH
2O in ethanolic HCl resulted in (
S)-(+)-Tylophorine.
Facile Approach to Enantiomerically Pure ?-Amino Ketones by Friedel-Crafts Aminoacylation and Their Conversion into Peptidyl KetonesMaria Luisa Di Gioia, Antonella Leggio, Angelo Liguori, Anna Napoli, Carlo Siciliano, and Giovanni Sindona
J. Org. Chem.,
66(21), 7002-7007 (2001).
DOI:
10.1021/jo010414q


See also
Post 475692
(Rhodium: "A two-step method for chiral cathinones", Novel Discourse).