A One-Step Ester to Hydrocarbon ReductionDonald C. Wigfield and Kevser TaymazTetrahedron Letters 49, 4841-4844 (1973)Oxidation and reduction constitute an important part of organic chemistry, and such reactions of oxygenated functional groups occupy a central place in this area, with many reactions having been developed to transform one functionality into another of differing oxidation level. The reduction of a Group III oxidation level
1 functional group (e.g. -COOR) to a Group 0 functional group (-CH
3) is, however, a transformation for which there is no general one-step reaction
2. Indeed, even the number of specific one-step transformations of this type that have been reported is rather small, the examples being apparently restricted to catalytic hydrogenation of certain substrates under vigorous conditions
3.
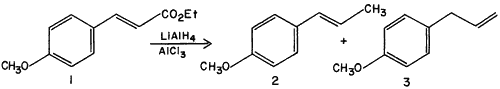
In view of the dearth of such reactions, we wish to report a reaction that we have discovered in the course of other work. Ethyl p-methoxycinnamate
1, on reduction with LiAlH
4-AlCl
3 in ether, gives not the expected p-methoxycinnamyl alcohol, but a quantitative yield of the two isomers anethole
2 and p-allylanisole (estragole)
3 in a 2:1 ratio (glc ratio 66:34). The identity of these compounds was established chromatographically using authentic samples (6 foot, 5% QF-1 on Chromosorb G column at 1400, anethole retention time 10.5 min., p-allylanisole retention time 4.7 min.) followed by preparative glc (QF-1) purification and demonstration of identity of spectral properties with those of authentic samples.
This reaction is, thus, an extremely rare instance of a one-step reduction of an ester to the corresponding hydrocarbon initiated by a metal hydride reagent
7. That it is by no means general, however, is immediately clear from the reduction of the closely related ethyl cinnamate, which has been reported to reduce normally to the primary alcohol under apparently identical conditions to ours
8. To ensure that this abrupt difference in reaction was due to the methoxyl substituent and was not an artifact of the comparison of results from different laboratories, ethylcinnamate was reduced, giving no hydrocarbon and producing cinnamyl alcohol in 95% yield, confirming the result of Jorgenson
8. Although at first sight the effect of methoxyl might be surprising since hydride reductions of ketones are known to show positive p values
9-11 presumably the reduction to the hydrocarbon involves Lewis acid-catalyzed
12 carbonium ion formation with a substantial negative
p value.
In a search of the literature to find closely related reactions we have found reports of certain alcohols being reduced to hydrocarbons under LiAlH
4-AlCl
3 conditions
13,14, and in a particularly relevant paper, the reductions of p-amino- and p-methoxybenzaldehydes to the corresponding toluenes, the electron donating substituents being essential for reduction to the hydrocarbon
15. Thus although the reaction at hand may at present be almost unique, its existence might not have been entirely unpredictable, the possibly surprising feature being the quantitative ester reduction accompanied by no detectable double bond reduction.
In order to explore the limited generality of the reaction, reduction of the corresponding aldehyde and alcohol were also studied. p-Methoxycinnamyl alcohol, produced by LiAlH
4 reduction of methyl p-methoxycinnamate
16, also was reduced by LiAlH
4-AlCl
3 giving a quantitative yield of the anethole-estragole mixture in essentially the same proportions (64:36). p-Methoxycinnamaldehyde, produced by MnO
2 oxidation of p-methoxycinnamyl alcohol
16, was also reduced quantitatively to give the same hydrocarbon mixture (anethole:estragole 67:33).
Reduction of ethyl o-methoxycinnamate also gave quantitative reduction to hydrocarbon, the proportion of double bond isomers, however, being slightly different (o-anethole 56%, o-Estragole 44%).
The reduction in a system with an amino activating group, rather than methoxyl was also attempted. Reduction of ethyl p-aminocinnamate under the same conditions gave a complex mixture of products, from which the corresponding hydrocarbons p-allylaniline and p-propenylaniline
17 could be extracted only with tedious purification and in 18% yield.
References and Footnotes1. J. B. Hendrickson, D. J. Cram, and G. S. Hammond, Organic Chemistry. McGraw-Hill Book Company Inc., New York. 3rd Edition, 1970, p. 74.
2. Clearly there are many ways of accomplishing this transformation in more than one step.
3. P. N. Rylander. Catalytic hydrogenation over platinum metals. Academic Press, New York, 1967, pp. 230, 320, 476.
4. T. W. Campbell, S. Linden, S. Godshalk, and W. G. Young, J. Amer. Chem. Soc., 69, 880 (1947).
5. E. A. Braude, J. Chem. Soc., 1902 (1949).
6. A. E. Lutskii, A. F. Soldatova and E. M. Voroshin, Zh. Obshch. Khim., 35, 2099 (1965).
7. For another example, see H. O. House, Modern Synthetic Reactions. W. A. Benjamin, Inc., Menlo Park, California, 1972, p. 84.
8. M. J. Jorgenson, Tetrahedron Letters, 559 (1962).
9. J. A. Parry and K. D. Warren, J. Chem. Soc., 4049 (1965). 10. K. Bowden and M. Hardy, Tetrahedron, 22, 1169 (1966).
11. A. F. Cockerill and D. M. Rackham, J. Chem. Soc., Perkin II, 2076 (1972).
12. In this mixture, several species are possible, see U. E. Diner, H. A. Davis, and R. K. Brown, Can. J. Chem., 45, 207 (1967).
13. J. H. Brewster, S. F. Osman, H. O. Bayer, and H. B. Hopps, J. Org. Chem., 29, 121 (1964).
14. S. B. Nerali and K. K. Chakravarti, Tetrahedron Letters, 2447 (1967).
15. B. R. Brown and A. M. S. White, J. Chem. Soc., 3755 (1957).
16. D. Marshall and M. C. Whiting, J. Chem. Soc., 4082 (1956).
17. C. D. Hund and W. W. Jenkins, J. Org. Chem., 22, 1418 (1957).