Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5), http://www.mdpi.org/ecsoc-5.htm
, 1-30 September 2001 [E0008] http://www.mdpi.net/ecsoc-5/e0008/e0008.htm
Urea vs Thiourea in the formation of imides:
A Microwave and Conventional Heating Comparative Study
Julio A. Seijas*, M. Pilar Vázquez-Tato* and Carlos Álvarez-de-Gabriel
Departamento de Química Orgánica. Facultad de Ciencias.
Universidad de Santiago de Compostela. CAMPUS DE LUGO.
Aptdo. 280. 27080-Lugo. Spain
qoseijas@lugo.usc.es
,
pilarvt@lugo.usc.es
. Fax +34982224904
Received: 27 July 2001 / Uploaded 7 August 2001
Imide group is an interesting functionality, due to its wide presence in the natural products pool and in the pharmacologically active compounds. This has attracted our attention as a target to be prepared by microwave irradiation. We had published a general approach to N-substituted cyclic carboxylic imides [1], and later we extended our studies towards non substituted cyclic imides, thus we developed a new synthesis of thalidomide enhanced by microwave heating [2]. Thalidomide, an old drug with a renewed interest, has two imide groups in its structure. We carried out two microwave mediated new approaches: a stepwise synthesis by preparing first N-phthaloyl-L-glutamic acid and later using urea or thiourea (as the source of nitrogen) to close the glutarimide ring (scheme 1, right) and a one step reaction (scheme 1, left) from L-glutamic acid, phthalic anhydride and thiourea in the latter, obtaining with this procedure thalidomide in a 60% yield with the formation of the two imides simultaneously.
A feature that attracted very much our interest was the fact that when urea was used instead, we obtained a lower yield of thalidomide and a considerable amount of pyroglutamic acid. This, compared with the previous results for the formation of imides in the only study we know on urea and thiourea [3] resulted in a reversed reactivity.
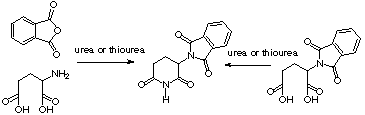
SCHEME 1
We decided to study independently the reaction of formation of phthalimide and glutarimide in the reaction conditions used for thalidomide, to check if there was any marked difference between formation of phthalimide and glutarimide rings (scheme 2) which were the responsible for the good behaviour in the preparation of thalidomide.
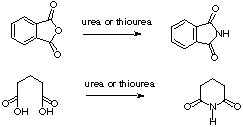
SCHEME 2
Thus we studied the formation of glutarimide by heating glutaric acid with urea and with thiourea. We found that after 15 minutes irradiation in the (domestic) microwave oven (Electrolux; 1000W, 70%) we obtained, 90% yield of glutarimide with urea, and 98% yield with thiourea. We also carried out both experiments in a preheated heating mantle (160ºC) for the same time as they were irradiated, getting a 50% yield for the reaction with urea and a 33% when thiourea was used.
The phthalimide formation from phthalic anhydride was studied in a similar way. The reaction with urea was irradiated for 6 minutes with a quantitative yield of phthalimide. Meanwhile, with the heating mantle it gave just 43% yield of imide. The use of thiourea with microwaves gave a lower yield of phthalimide (91%, and required 10 minutes irradiation), but with the heating mantle it was slightly higher (46%) than the conventional using urea.
Regarding to the improvement on the synthesis by microwave of our three component reaction for thalidomide, we think thiourea is less reactive than urea towards phthalic anhydride than urea, which could help to lessen the formation of phthalimide as a secondary product, and at the same time thiourea gives better yield in the formation of glutarimide ring.
In summary, for the same reaction times, microwave irradiation is the ideal choice for preparing non substituted cyclic imides. The use of urea or thiourea led to similar yields.
ACKNOWLEDGEMENTS DGES (PB96-0932) and XUNTA DE GALICIA (PGIDT01PXI26203PR) for financial support.
REFERENCES1.- Seijas, J. A.; Vázquez-Tato, M. P.; Martínez, M. M.; Nuñez-Corredoira, G.
J. Chem . Res. (S), 1999, 420-421. 2.- Seijas, J. A.; Vázquez-Tato, M. P.; González-Bande, C.; Martínez, M. M.; López-Pacios, B.
Synthesis, 2001, 999-1000. 3.- Rahman, A. U.
Recl. Trav. Chim. Pays-Bas, 1956, 75, 164-168. Rahman, A. U.; Medrano, M. A.; Mittal, O. P.,
ibid. 1960, 79, 188-192.