Here's a procedure that may interest tube furnace bees. It is taken from
Fundamental Processes of Dye Chemistry, 5th edition, which I will soon make available in full.
Phthalic Anhydride from Naphthalene by Catalytic Oxidation (see German patents 347,160 and 379,822; see also Chowdhury and Chowdhury, J. Indian Chem. Soc., 11, 185(1934), and Chowdhury and Saboor, ibid, 14, 638 (1937.))
The heat of combustion of naphthalene is +1234 kilocalories per mole, and of phthalic anhydride, +450 kilocalories per mole. The catalytic oxidation of naphthalene can easily be carried out in the laboratory, although the amount of phthalic anhydride which can be prepared in one operation is insignificant. It is of great importance that the correct temperature be maintained and that a suitable catalyst be used. Special attention must be given to the apparatus if the preparation in the laboratory is to succeed. Furthermore, it is highly desirable to use a Cottrel precipitator to collect the reaction product completely. This apparatus will collect even the fine particles, which otherwise would be lost.
Preparation of the CatalystPumice is broken up into pieces about 3 mm. in size and screened, using a sieve that retains the 3-mm. pieces. The more uniform the pieces are, the better. This pumice is placed in a hot solution of ammonium vanadate which is as concentrated as possible. When the pumice is thoroughly impregnated, it is removed from the solution and dried in a porcelain dish on a water bath. About 3 grams of vanadium are taken up by 200 grams of pumice. The dried mass is now heated to 400°C. until the grains have a reddish brown color. It is important that the catalyst is not heated above the temperature at which it is to be used later, a precaution to be observed in many catalytic reactions.
Rule: A catalyst should not be heated above the reaction temperature.Performing the Reaction, ApparatusThe diagram in Figure 28 best explains the procedure. Naphthalene is placed in flask B, which is heated to 110°C., and a stream of moist air is passed through the gas introduction tube (A). The presence of water vapor is important for the oxidation. The necessary air is most simply supplied by a water pump which is readily available in the laboratory, and the quantity of air passed through the apparatus is measured with regular laboratory equipment which need not be described here. The air stream should be regulated so that 200 liters of air and 5 grams of naphthalene are passed through the apparatus in 1 hour. It is important that the ratio of air to naphthalene be controlled so that an explosive mixture is not formed, since this would cause a loss of phthalic anhydride. The mixed gases (air and naphthalene vapor) are passed into the catalyst tube, which should be of about 5-cm. internal diameter and should contain a charge of catalyst about 10 cm. in length. The pumice particles are held in place by glass wool. Heating of the catalyst is done electrically as is the usual practice in technical laboratories.
The gases coming from the catalyst are passed first into a round receiver (J), and then go to the precipitator. The latter consists of a glass tube of about 8-cm. internal diameter, the inside wall of which is lined with a smooth aluminum foil which can be removed easily and which is well grounded through a metal wire to a water pipe. A wire or silvered glass rod projects into the tube, and to this a potential of 10,000 volts is applied. This potential, which can be alternating for the present purpose, is generated in the usual manner, most simply by an induction coil or a transformer with interrupter. It is important that the air spark length of the induction coil be at least 25 mm. The internal width of the tube carrying the precipitator must therefore be sufficiently great so that sparks do not jump from the wire to the metal foil. Such sparking may cause explosions which, although not involving danger, may easily burn part of the reaction product.
When the apparatus, to which careful attention must be given, is assembled correctly, the oxidation can be started. The catalyst tube is heated to 450°C. (thermocouple), and the stream of mixed air and naphthalene vapor is started. The oxidation takes place as the vapors pass through the catalyst. Part of the phthalic anhydride collects in the bulb receiver, but most of it is collected in the Cottrel precipitator where it frequently forms beautiful needles, up to 5 cm. in length, in a small space at the extreme first part of the precipitator. The operation can be continued as long as desired, since the catalyst retains its activity for weeks provided that pure naphthalene is used. The yield of phthalic anhydride is about 90 to 95 per cent of the theoretical amount, the product being chemically pure if the oxidation temperature is maintained at the correct point and the throughput is not too high. If too much naphthalene is put through per unit of time, the product becomes yellowish due to admixed 1,4-naphthoquinone. The product is removed from time to time by drawing out the aluminum foil. 20 grams of pure phthalic anhydride can easily be prepared in 5 hours in the laboratory.
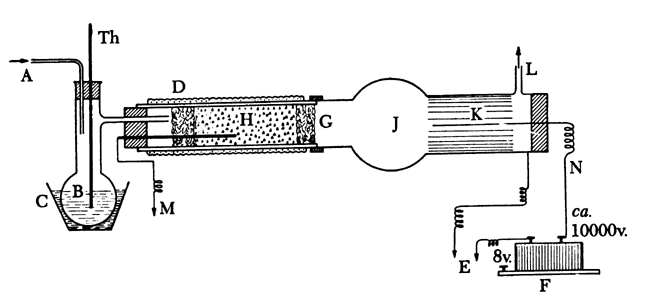
Fig. 28. Apparatus for preparation of phthalic anhydride by catalytic oxidation:
A, air inlet tube; B, melted naphthalene; C, graphite bath; D, electrical heating element; E, ground; F, spark induction coil; G, glass wool; H, catalyst; J, bulb receiver; K, aluminum foil, grounded; L, gas exit tube; M, thermocouple; N, rigid wire; Th, thermometer. One side of the spark induction coil is connected to N, the other is grounded.