PERACID INFO: for Newbees and bees alike! As this seems to be a topic of relevancy at the moment SWIM thought that he would add SWIM's contribution to the HIVE'
Hydrogen peroxide applications in organic chemistry
Hydrogen peroxide is used in many reactions in organic chemistry ; among them the most important ones are :
synthesis of peroxides (peracids, hydroperoxides, diacyl peroxides…) ;
epoxidation of olefins ;
oxidation of amines.
Synthesis of peroxides:
The peracids 'see
https://www.thevespiary.org/rhodium/Rhodium/chemistry/peracid.html
(peracetic acid, perpropionic acid) are produced by oxidation of the corresponding acids by H2O2 in presence of an acidic catalyst. These reactions are governed by a chemical equilibrium, peracid content at the equilibrium is much higher when H2O2 used is more concentrated. Furthermore, another means to shift this equilibrium in order to obtain anhydrous solutions of peracid consists of eliminating water by azeotropic distillation (heteroazeotrope solvent/water) as it is formed.
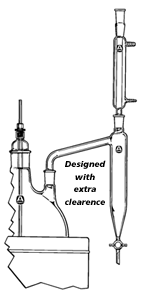
Designed specifically for azeotropic distillation using solvents lighter than water. Each Dean-Stark trap comes standard with a 4mm bore PTFE stopcock. Of course, there are many varieties concerning this application, but this provides one with the basic gist of the application.
The hydroperoxides may be prepared by oxidation of the corresponding alcohols by means of H2O2 in presence of an acidic catalyst (for example H2SO4).
Finally, diacyl peroxides can be synthesized from the corresponding acid chloride, H2O2 and caustic soda.
Epoxidation of double bond C=C:
Different routes are available to carry out epoxidation of double bond C=C : epoxidation by hydroperoxides, epoxidation by peracids and epoxidation by H2O2 catalyzed by zeolites.
Epoxidation by peracids may be performed either by a peracid prepared in a preliminary step (see previous paragraph concerning "synthesis of peracids"), or by a peracid prepared in situ. A process of this latter type consists of using simultaneously H2O2, formic acid, an acidic catalyst and the material to epoxidize.
Amine oxidation:
Amines react with hydrogen peroxide differently regarding the type of amine (primary, secondary, tertiary) and according to the operating conditions.
Primary amines lead to the formation of either hydroxylamines or oximes or nitroalcanes. Secondary amines are oxidized mainly in N-substituted hydroxylamines.
For tertiary amines, the chemistry is simpler because only the corresponding amine oxides are formed
INFO: For newbees and bees alike, like my self!
