Hello there fellas!
i found some great news for ya....a new way of making HI that looks really simple!
While searchin between different websites for information on Hydriodic acid i found this revolutionary synthesis.
Making HI from decahydronaphthalene(DECALIN)!!
ok in this reaction the iodine gets converted into HI directly from the alkenation of decahydronaphthalene(an aromatic carbon) via a catalyst!
the reaction goes like this:
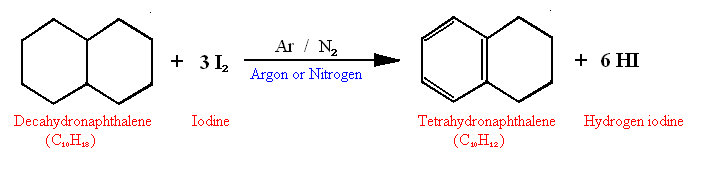
simple heh?
C10H18 + 3 I2 ---Ar/N2---> C10H12 + 6 HIIn this reaction 3 moles of iodine reacts with one mole of Decalin thanks to an argon or nitrogen catalist to conver the firts cyclohexane ring into a benzene ring(tetrahydronaphthalene).
wow.....looks promising! aromatics are not that hard to get since they r used in many different products since its a solvent!
Unfortunately i havent tested the reaction yet and i found only one source of info so far....but that sourse is reliable guys..i got it from the biggest chemestry website of germany(Chemie.de) in a page where they explain all different methods to get HI and there it was.
Well....i love chemestry but im not that good in it....like i still dont noe why certain reactions occur...all those alpha,beta things i still dont understand...but im pretty sure this reaction is tru since i read it in such an important website.
Now decahydronaphthalene is a common aromatic hydrocarbon that for wut ive read its used in many products that involve solvents. im sure it wont be so hard to find a source!
anyway Decalin must be treated with care...there are a few rules to follow while working with it.
So.......ur great Buddy NaVaRoNe made a wonderful Decalin chemical info table for you!
Here it is guys:

Notes the different Propeties!
Decalin decomposes at 57c so it must be treated with not too much heat!
note also that in the info i aquired..it sais that its used in many common products.
Wow this reaction looks good!
hmmm but now lets think of the setup for this reaction:
i already prepared something that should do perfectly the trick i guess.
wut do u guys think about this setup?
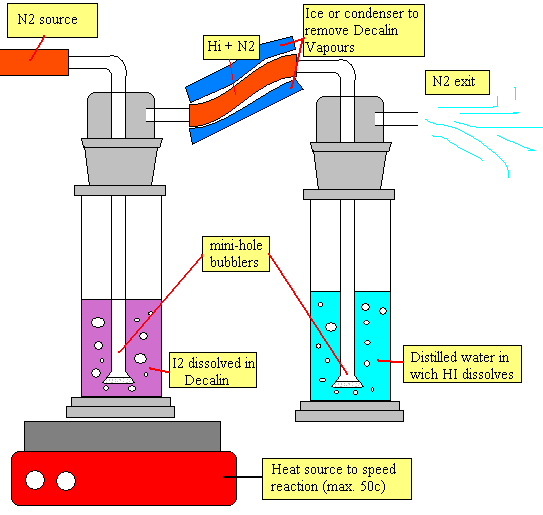
so here we use 2 gassing apparatuses with 2 foam bubblers(needed to have a calm soft bubbling and to help the HI dissolve better...its a matter of physics).
ok..in the first gassing apparatus a nitrogen source like a nitrogen tank or an argon tank is connected to the first bubler going down to the decalin iodine 1/3 molar solution.
the foam bubler hels the liquid not to splash and slows down the nitrogen bubbles helping the reaction between decalin and iodine. the first gassing bottle is gently kept under heat to speed the reaction.....but it must not go over 50c to prevent decomposin of the decalin and too many vapours in flask 2.
when the gasseous HI is formed(wich is not soluble in decalin) it goes up to the exit tube connected to the next gassin flask. a cooling source is reccomended to avoid decalin vapours going into flask 2 and giving a nice dry HI N2 gas.
this following gas goes down the bubler where it will spread into small bubbles into the distilled water thanks to the foam. the small bubbles are easier to dissolve quickly in the distilled water.
if the bubbles are small...sure all the HI is gonna dissolve into the H20 and the unreactive N2 gas escapes from the top exit hole.
the reaction should be visible since the decalin iodine solution in flask one should loose its purple color thanks to the iodine conversion into HI.
when the liquid in flask one is completely colourless..then all the iodine has been converted into HI and dissolved in flask 2 giving the wonderful hydriodic acid!!

Just keep on bubbling your distilled water in flask 2 until u have the right concentration of HI.
heheeh....wut do u think?
anyway i was thinking about using the decalin as a recycling agent in the meth reaction....maybe attachig a dropping funnel on the reaction flask and dropping the decalin in slowly.
but since im not a chemestry expert..i still dont noe if the decalin(decahydronaphthalene) or the tetralin(tetrahydronaphthalene from the reaction with iodine) will interfear the reaction.
one thing is for sure...the decalin and tetralin will float on the reaction since they r non polar.
can anybody temme if decalin or tetralin will mess up the reaction....please.
well well it looks promising...but could anybody of you put your comments please.....im not that chem genious and i want to hear the opinion of real experts.
and yes..another thing........if anyone here has the chemicals to attemp this reaction..please try it out and post all the results!
well hope this is useful for you guys!
peace yo
-NaVaRoNe-