
|
 |
|
|
|
Impurities in illicit amphetamine: a reviewSectionsABSTRACT DetailsAuthor: A. SINNEMA, A. M. A. VERWEIJ Impurities in illicit amphetamine: a reviewA. SINNEMA Laboratory of Organic Chemistry, Department of Chemical Technology, Delft University of Technology, Delft, the NetherlandsA. M. A. VERWEIJ Forensic Science Laboratory of the Ministry o f Justice, Rijswijk, the Netherlands ABSTRACTThe paper reviews the most common synthesis of amphetamines which may be found in illicit traffic. Emphasis is laid on the detection, isolation and identification of impurities in illicit amphetamines through gas chromatography and thin-layer chromatography. The latter method was also used for isolation purposes. Impurities were identified by mass spectral and NMR spectroscopic methods and relevant data are presented. IntroductionIllegally produced drugs are frequently contaminated, as is readily shown by chromatographic analysis. Contaminations in drugs can be caused by laboratory diet, impurities in the starting chemicals, side- and subsequent reactions, intermediate products, diluents of the drugs and from handling and packing of the drugs. Hence two different approaches can be used for their identification:
In this review, attention will be focused on the latter approach, since chemical compounds present in the final amphetamine, due to inadequate purification of the raw product, will give valuable clues to the method of synthesis. In this way, suspected illicit sources of amphetamine can be confirmed and information provided to the police which may contribute to tracking down the illegal production locations. Synthesis of amphetamineTo date, 23 clandestine laboratories have been discovered in the Nether-lands [1] . The Leuckart reaction (see figure I) [2] was the most frequently encountered, though in some cases reductive amination of benzyl methyl ketone was found (figure II). No other syntheses such as the oxime or the phenylnitropropene routes were encountered. Only once was a methamphetamine production location found; in this instance the substance was prepared by an almost professional procedure [3] . Our findings therefore contrast with those reported in the United States of America, where the accent seems to be on the production of methamphetamine [4] . Most of the amphetamine was intended for the markets of the Federal Republic of Germany and Scandinavia; only a minor portion of it was sold in the Netherlands. Figure IThin-layer chromatogram of weakly basic fraction on Merck precoated silica gel 60 F-254 with hexane/ether (50+50) as eluent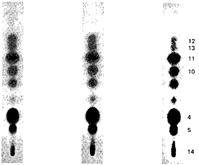 4 4-methyl-5-phenylpyrimidine 5 5-benzylpyrimidine 9 2,4-dimethyl-3,5-diphenylpyridine 10 2,6-dimethyl-3,5-diphenylpyridine 11 4-methyl-5-phenyl-2-(phenylmethyl)pyridine 12 2-methyl-3-phenyl-6-(phenylmethyl)pyridine 13 2,4-dimethyl-3-phenyl-6-(phenylmethyl)pyridine 14 2-benzyl-2-methyl-5-phenyl-2,3-dihydropyrid-4-one Compounds 4 and 5 were stained yellow by spraying the spots with 1% p-dimethylamino-benzaldehyde in 50% sulphuric acid and heating the plate at 100°C in a drying box. The three pyridine derivatives with no &alpha-hydrogens were stained blue-purple with iodoplatinate reagent, the other two pyridines and pyrimidines became yellow or brown. With the latter reagent the pyridone turns reddish. Figure IIComputer reconstructed chromatogram of the low-pressure, Iow-temperature reductive amination reaction mixture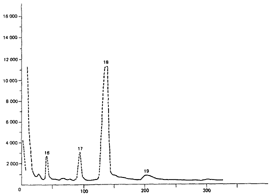 Impurities in illicit amphetamine: a review 39 16 N-acetylamphetamine 17 N-(&beta-phenylisopropyl)benzaldimine 18 N-(&beta-phenylisopropyl)benzyl methyl ketimine 19 1-oxo-1-phenyl-2-(&beta-phenylisopropylimino)propane Chromatographic conditions were as follows: 2 metre s.s. (&empty 1/8"), column packed with Apiezon/KOH 10%/10% on Chromosorb G DCMS (80-100 mesh), oven temperature 220°C, carrier gas He, flow rate 10 ml/min, The Leuckart reactionThis reaction can be described by the following scheme: 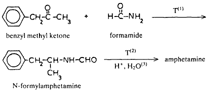 in which (1): 180-195°C. (2): 90-125°C. (3): H 2SO 4, HCl (diluted). Reaction conditions can vary [5] , formamide being sometimes replaced by ammonium formate [6] or a mixture of ammonia and formic acid [7] . It is clear that, due to the many possibilities for condensation of benzyl methyl ketone with formamide, a host of other products can be expected. This gives rise to a large number of impurities in the more or less purified amphetamine. In practice, approximately 50% amphetamine is recovered [8] ; with the formic acid variant, recovery of about 30% is feasible [9] . Methamphetamine prepared according to this method contains fewer impurities as the N-methylformamide, used as starting material, has fewer possibilities for condensation with benzyl methyl ketone. 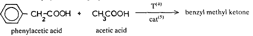 Benzyl methyl ketone may be purchased from wholesale dealers, but can also be easily synthesized [10] . 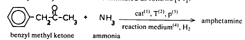 in which (4): 450°C. (5): THO 2. Other methods have also been reported [11-13] . Dibenzyl ketone is a side-product of this reaction and its presence in amphetamine is readily understood if benzyl methyl ketone is produced in this way. The reductive amination of benzyl methyl ketoneBenzyl methyl ketone can be aminated as follows [ 14] : in which (1): Raney-Ni, Pt, A1 powder in the presence of HgCl 2, nickel plated Zn. (2): 20-170°C. (3): 1-130 Atm. (4): Ethanol or methanol. Reaction conditions reported in the literature differ widely [ 15- 18]. To date, only low-pressure and low-temperature aminations have been found in this country. Recoveries of approximately 90% can be reached if optimized reaction conditions are carefully maintained and freshly prepared Raney-nickel used [19] . In this case there are fewer impurities than in the Leuckart route. The oxime routeUpon reaction with hydroxylamine, benzyl methyl ketone gives the oxime, which can subsequently be hydrogenated to yield amphetamine [20]. 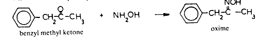 in which (1): 20- 170°C. (2): 1-130 Atm. (3): Na (amalgamated), Na (absolute ethanol), LiAlH 4, or H 2 and Raney-Ni or Ni or Fe or nickel plated Zn. Great differences are reported in the reaction conditions [21-23]. Electro-lytical reduction has also been described [24] . However, this route has to our knowledge not been put into practice for the illegal production of amphetamine. Recoveries of up to 90% have been reported. The phenylnitropropene routeCondensation of benzaldehyde with nitroethane yields l-phenyl-2-nitro-propene. Hydrogenation of the double bond and reduction of the nitro-group give the amphetamine. 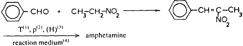 in which (1): 20- 100°C. (2): 1-80 Atm. (3): LiAlH 4, H 2 and Raney-Ni or Pd/C. (4): CH 3OH; C 2H 5OH; H 20/HCOOH; CH 3COOH/C 2H 5OH. Reaction conditions vary widely [25, 26] and electrolytical reduction has been mentioned [27] . This synthesis has been of importance in Sweden in illegal amphetamine production [28] . We have given a rather extensive description of some methods for the production of illegal amphetamine for which the starting material can be purchased easily and in which the chemical operations are not too complicated. Many other synthetic routes for amphetamine, often with obscure or intricate chemicals, are known,1but in this context are of lesser importance. 1. A literature search is available for the interested reader. Detection, isolation and identification of impurities in illicit amphetamineTo obtain impurities in sufficient quantities for analysis, the raw material was diluted with water and acidified with tartaric acid. This solution was extracted with ether, and the ether layer was extracted with 4N hydrochloric acid. A portion of the hydrochloric acid solution was made alkaline and extracted with chloroform to give fraction A (weak bases). A quantity of the ether layer was evaporated to give fraction B (neutral substances). Likewise, the tartaric acid solution was made alkaline and extracted with chloroform to give fraction C (strong bases). All three fractions were investigated, but our attention was directed mainly to the weak bases. For a quick survey of the impurities present, thin-layer chromatography is the method of choice. Several eluents, e.g. cyclohexane-benzene-diethylamine (75 : 15 : 10), hexane-acetone (50 : 50) or (80 : 20), hexane-ether (50: 50) or (90 : 10) and methanol-ammonia 25 % (99 : 1) were tried, of which hexane-ether (50 : 50) gave the best results (see figure I). Gas chromatography on 2% OV-17 or 10%/10% Apiezon/KOH columns in an isothermal (200°C) or better, in a programmed mode (100-250°C, 2°C/min) was performed (see figure II). Isolation of the compounds with a purity of better than 95% can be achieved by repetitive thin-layer chromatographic runs, using a combination of the above-mentioned eluents. In the literature, a high-performance liquid chromatographic (HPLC) method for these purposes has been reported [28] . The isolated compounds were identified by a combination of low- and high-resolution mass spectrometry, and [1] H and [1#3] C NMR spectroscopy [29-34]. None the less, the described isolation procedure does not always give the desired results. The gas chromatograph/mass spectrometer combination can then be used in the low- and the high-resolution mode. Compounds, whose structures were tentatively derived from mass spectral data, were synthesized. In most instances the problem of structure elucidation was then solved by comparison of the chromatographic and the mass spectral data of the synthesized substances with those whose structures are to be elucidated [35, 36]. The use of low-resolution mass spectral data alone in structure determination is not recommended. It can easily lead to erroneous results because the mass spectra of the various compounds can be very similar. Impurities found in illicit amphetamine prepared via the various synthetic routesThe impurities found so far in the Leuckart synthesis and the reductive amination routes are presented in tables 1 and 2. Remarkably few impurities are known of the other two routes. Str?mberg reported [28] the presence of phenylacetoxime in a seizure of amphetamine prepared by the electrolytic reduction of phenylnitropropene, while Kotera [37] has indicated 2-methyl-3-phenylaziridine and 2-benzylaziridine as by-products in the reduction of phenylacetoxime with lithium aluminium hydride. Table 1Reported impurities in amphetamine synthesized by the Leuckart method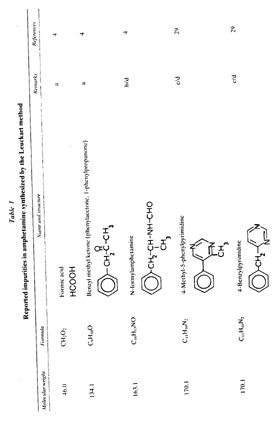
Table 1 (continued)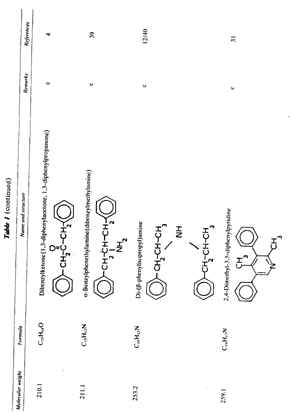
Table 1 (continued)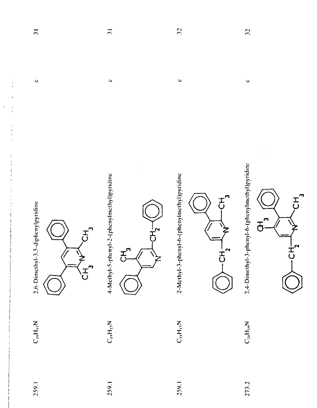 Note: a = intermediate product: b = product of side reaction. Table 2Reported impurities in amphetamine synthesized by low-pressure and low-temperature reductive animation.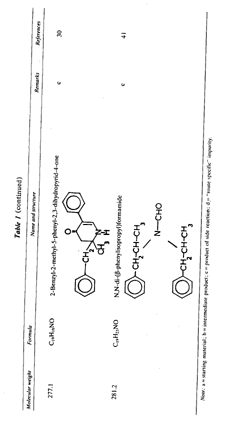
Table 2 (continued)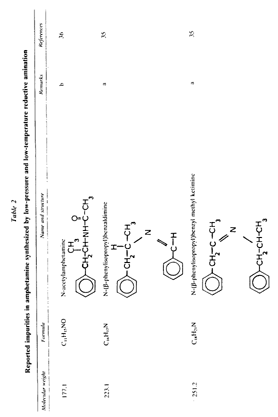
Mass spectrometric and 2 H NMR spectroscopic dataIn table 3 the mass spectral fragmentations are given for the compounds described in tables 1 and 2 (m/e of the most intense fragments are in decreasing order of intensity). In table 4, 1H NMR spectroscopic data are given for most of the impurities in tables 1 and 2. Table 3Mass spectral data of impurities (m/e of most intense fragments in decreasing order of intensity)
a. The actual mass spectrum is of the dehydrated compound. Note: B = base peak. In most cases 70 e V electron impact spectra are given. Table 4: 1 .H NMR spectroscopic data and assignments for most of the impurities
Note. s = singlet; d = doublet; m = multiplet; ss = double singlet; &psi-s = pseudo-singlet; bs = broad singlet; AB = a proton pair for which the difference in chemical shift is of the same order as the coupling constant; J = coupling constant. a. A double singlet, with unequal intensities, is observed from two isomers due to restricted rotation about the C-N bond. b. The data are for the keto-form, which dominates in solution. ConclusionsAmong the impurities identified so far in illicit amphetamine produced by the Leuckart synthesis, some compounds such as N-formylamphetamine, 4-methyl-5-phenylpyrimidine and 4-benzylpyrimidine can be designated as "route specific" [38] , i.e. by their presence they give direct evidence of the synthesis used. In particular 4-methyl-5-phenylpyrimidine is extremely well suited for this purpose as we found it present in high quantities in nearly all illicit amphetamines analysed. Thin-layer chromatography, preceded by the extraction described above, is the method of choice at this point in the analysis. The use of the Leuckart route can then further be established by looking for some of the other impurities mentioned in tables 1 and 2. This should be done by the gas chromatographic/mass spectrometric method in the low-resolution mode, making use of the mass spectral data in table 3. The variation in mass spectral data between classes of compounds is specific enough to ascertain the class. Within a class the various compounds must first be isolated and then structure elucidation is straightforward by NMR spectroscopic methods. Impurities in illicit amphetamine: a review 51The art of Iow-pressure, low-temperature reductive amination seems somewhat underdeveloped as compared with the Leuckart route. The impurity, 2,4-dihydroxy-l,5-diphenyl-4-methylpentene-1, was incidentally found to originate in our opinion from imperfect chemical handling. Of the other five impurities in table 2, Haskelberg [14] already mentioned di-(&beta-phenyliso-propyl)amine as an impurity in reductive amination (the compound is already known from the Leuckart route). The intermediate product N-(&beta-phenyliso-propyl)benzyl methyl ketimine is, to our knowledge, present in fair quantities in all amphetamines produced by this method. It was pointed out, however, that N-(&beta-phenylisopropyl)benzyl methyl ketimine together with N-acetylamphet-amine, N-(&beta-phenylisopropyl)benzaldimine and 1-oxo-l-phenyl-2-(&beta-phenyl-isopropylimino)propane can also be obtained by refluxing equivalent amounts of amphetamine and benzyl methyl ketone in benzene [34, 35]. The presence of N-(&beta-phenylisopropyl)benzyl methyl ketimine on its own by no means gives substantial evidence of the reductive amination route to amphetamine. The simultaneous presence of di-(&beta-phenylisopropyl)amine and N-(&beta-phenyliso-propyl)benzyl methyl ketimine separated by gas chromatography using capillary columns and then identified by mass spectroscopy using the difference between their molecular ions m/e 253 and m/e 251 and the abundant m/e 162 and m/e 160 ions, leads us to conclude that a hydrogenation step at least was involved. This evidence of organic impurities may possibly be supported by a higher level of inorganic impurities from the catalyst used in the production of amphetamine. Due to scarcity of published data no further remarks about the oxime and phenylnitropropene route are given here. AcknowledgementThe authors thank Dr. A. M. van der Ark for stimulating discussions and Ing. A. B. E.Theeuwen and Mr. I. M. van der Toorn for their skilful assistance in the experimental part of this work. References001H. Huizer, personal communication (1981). 002R. Leuckart, "?ber eine neue Bildungsweise von Tribenzylamin", Berichte der Deutschen Chemischen Gesellschaft, vol. 18, 1885, p. 2341. 003A.M. van der Ark, A. B. E. Theeuwen and A. M. A. Verweij "Verunreinigungen in illegalem Amphetamin 6. Identifizierung von Phenylpropanok Amphetamin und N 1 N 1-Dimethylamphetamin in Methamphetamin", Archiv f?r Kriminologie , vol. 162, 1978, p. 171. 004"Review of illicit traffic in narcotic drugs and psychotropic substances during 1979" (E/CN.7/660 (Part one)), para. 34. 005M. L. Moore, "The Leuckart reaction", in Organic Reactions (New York, John Wiley, 1949), vol. V, chapter 7, p. 301. 006O. Wallach, "?ber Menthylamin", Berichte der Deutschen Chemischen Gesellschaft , vol. 24, 1891, p. 3992. 007F.S. Crossley and M. L. Moore, "Studies on the Leuckart reaction", Journal of Organic Chemistry , vol. 9, 1944, p. 5291. 008I.B. Johns and J. M. Burch, "The synthesis and resolution of alpha-o-chlorobenzyl-ethylamine", Journal, American Chemical Society , vol. 60, 1938, p. 919. 009A. Novelli, "Sympaticomimetics. Preparation of nitrogen substituted &beta-phenyliso-propylamines", Anales de la A.sociacion Quimica (Argentina), vol. 27, 1939, p. 169. 010R. M. Herbst and R. H. Manske, "Methyl benzyl ketone", in Organic Synthesis 16 (New York, John Wiley, 1936), chapter XVI, p. 47. 011B. Radiziszewski, "Zur Geschichte der Phenylessigs?ure", Berichte der Deutschen Chemischen Gesellschaft , vol. 3, 1870, p. 198. 012S. Young, "Dibenzyl Ketone", Journal of the Chemical Society , vol. 59, 1891, p. 621. 013A. Popoff, "Oxydationsprodukte der Benzylketone", Berichte der Deutschen Chemischen Gesellschaft , vol. 5, 1872, p. 500. 014L. Haskelberg, "Aminative reduction of ketones", Journal, American Chemical Society , vol. 70, 1948, p. 2811. 015P. L. Couturier, "Action des d?riv?s organomagn?siens mixtes", Annales de Chimie , vol. 10, 1938, p. 559. 016E. R. Alexander and M. L. Misegades, "A low pressure reductive alkylation method for the conversion of ketones to primary amines", Journal, American Chemical Society , vol. 70, 1948, p. 1315. 017B. H. Groot-Wassink, A. Duyndam and A. C. A. Janssen, "Synthesis of amphetamine'', Journal of Chemical Education , vol. 51, 1974, p. 671. 018E. J. Schwoegler and H. Adkins, "Preparation of certain amines", Journal, American Chemical Society , vol. 61, 1939, p. 3499. 019H. Huizer and A. J. v. d. Meer, "A new method in illegal amphetamine production", Microgram, vol. 8, 1980, p. 118. 020D. H. Hey, "dl-&beta-Phenylisopropylamine and related compounds", Journal of the Chemical Society , 1930, p. 18. 021Purdue Research Foundation, "Process for the reduction of arylnitroalkenes", United States Patent 2.233.823, 1939. 022F. M. Jaeger and J. A. van Dijk, "Preparation of 2-phenylisopropylamine (benze-drine) the isomeric 1-phenylpropylamine and 3-phenyl-1,2-propanediamine and the resolution of these bases into their optical antipods", Proceedings of the Section of Sciences of the Koninklijke Academie van Wetenschappen (Amsterdam), vol. 44, 1941, p. 26. 023E. Larsen, "Notiz fiber die Reduktion von Oximen mit Lithium-aluminiumhydrid", Svensk Kemisk Tidskrift , vol. 61, 1949, p. 242. 024F. Nabenbauer, "Method for the production of substituted benzyl carbinamines and products thereof", British Patent 447.792, 1936. 000Impurities in illicit amphetamine: a review 53 025Commercial Solvents Corporation, "Catalytic reduction of nitro olefins", United States Patent 2.647.930, 1953. 026Great Lakes Carbon Corporation, "Reduction of arylnitroalkenes", United States Patent 3.458.576, 1969. 027G. A. Alles, "dl-Beta-Phenylisopropylamines", Journal, American Chemical Society , vol. 54, 1930, p. 271. 028L. Str6mberg and A. C. Maehly , Proceedings of the International Symposium on Instrumental Applications in Forensic Drug Chemistry (Washington D.C., United States Department of Justice, 9781, p. 202. 029A.M. van der Ark, A. B. E. Theeuwen and A. M. A. Verweij, "Impurities in illicit amphetamine 1. Isolation and identification of some pyrimidines", Pharmaceutisch Weekblad , vol. 112, 1977, p. 977. 030A.M. van der Ark and others, "Impurities in illicit amphetamine 2. Isolation and identification of 2-benzyl-2-methyl-5-phenyl-2,3-dehydropyrid-4-one", Pharmaceutisch Weekblad , vol. 112, 1977, p. 980. 031A.M. van der Ark and others, "Impurities in illicit amphetamine 3. Isolation and identification of 2,4-dimethyl-3, 5-diphenyl pyridine, 2,6-dimethyl-3,5-diphenyl pyridine and 4-methyl-5-phenyl-2-(phcnylmcthyl)pyridine", Pharmaceutisch Weekblad , vol. 113, 1978, p. 41. 032A.M. van der Ark and others, "Impurities in illicit amphetamine 4. Isolation and identification of 2-methyl-3-phenyl-6-(phenylmethyl)pyridine and 2,4-dimethyl-3-phenyl-6(phenylmethyl)pyridine", Pharmaceutisch Weekblad , vol. 113, 1978, p. 341. 03333, A.M. van der Ark, A. M. A. Verweij and A. Sinnema, "Weakly basic impurities in illicit amphetamine", Journal of Forensic Sciences , vol. 23, 1978, p. 693. 034H. Huizer anti others, "Impurities in illicit amphetamine 8", Journal of Forensic Science Society , in press. 035A. B. E. Theeuwen and A. M. A. Verweij, "Impurities in illicit amphetamine 7. Identification of benzyl methyl ketone phenylisopropylimine and benzyl methyl ketone benzylimine in amphetamine", Forensic Science International , vol. 15, 1981, p. 237. 036A. B. E. Theeuwen and A. M. A. Verweij, "Verunreinigungen in illegalem Amphetamin 9. ldentifizierung yon N-Acetylamphetamin und l-oxo-l-phenyl-2(phenylisopropylimino)propan", Archiv f?r Kriminologie , in press. 037K. Kotera and others, "Aziridine formation by reduction of ketoximes with lithium-aluminiumhydride. Dibenzylketoxime and its O-substituted derivations", Tetrahedron, vol. 24, 1968, p. 6177. 038D.G. Sanger, I. J. Humphreys and J. R. Joyce, "A review of analytical techniques for the comparison and characterization of illicit drugs", Journal of Forensic Science Society , vol. 19, 1979, p. 65. 039T.C. Kram, "Identification of an impurity in illicit amphetamine tablets", Journal of Pharmaceutical Sciences , vol. 66, 1977, p. 443. 040J.N. Lamonte, W. T. Lowry and I. C. Stone, "Contaminants in illicit amphetamine preparation", Journal of Forensic Sciences , vol. 21, 1976, p. 575. 041T. C.Kram, "Reidentification of a major impurity in illicit amphetamine", Journal of Forensic Science , vol. 24, 1979, p. 596. 042E. Stenghagen, A. Abrahamsson and F. W. McLafferty, Atlas of Mass Spectral Data (New York, Interscience, 1969), vol. 1.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|