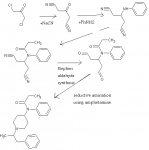
Aight, I didn't find an appropriate thread for this, so here goes:
Acetone-->1,3-Dichloroacetone (There's a patent on this using iodides)--NaCN-->1,3-Dicyanoacetone-->reductive amination with aniline-->amide by acetic or propionic anhydride-->dialdehyde (by Stephen aldehyde synthesis, my coup de grace) --reductive amination with phenylethylamine-->fentanyl or its derivatives
Any obvious mistakes I didn't get? Any thoughts?
Helgoland
Acetone-->1,3-Dichloroacetone (There's a patent on this using iodides)--NaCN-->1,3-Dicyanoacetone-->reductive amination with aniline-->amide by acetic or propionic anhydride-->dialdehyde (by Stephen aldehyde synthesis, my coup de grace) --reductive amination with phenylethylamine-->fentanyl or its derivatives
Any obvious mistakes I didn't get? Any thoughts?
Helgoland