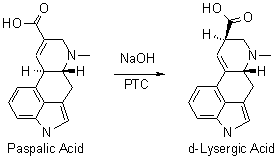
of course, in the known scheme the PTC is tetrabutylammonium hydroxide.
could a quaternary ammonium hydroxide be substituted with a quaternary ammonium chloride, such as adogen 464?









Choline hydroxide is one of the class of phase transfer catalysts that are used to carry the hydroxide ion into organic systems, and, therefore, is considered a strong base. It is the least costly phase transfer catalyst, and is used as a cheap method of stripping photoresists in circuit boards. Choline hydroxide is not completely stable, and it slowly breaks down into trimethylamine.









 .
. 


























Yes but can Cl2 even truely exist in an (Aq) solution? Is should form HCl and HOCl on contact with water the later which I would think should cause havoc on most organic substances let alone something as unstable as LSD.It's an equilibrium that greatly favors aqueous chlorine over HOCl and HCl