It has come to my attention that the D isomer of methylamphetamines and amphetamines are the more active compounds, however, I believe it to be true that L-Tryptophan is the more potent of the two isomers.
Don't take my word on it, but a book on medicinal chemistry says "the R isomer is more potent in tryptamines", which is the L-tryptamine, right? I seemed to have lost my confidence in such matters.
I will expand on this later. Much too much to think about at the moment.
Don't take my word on it, but a book on medicinal chemistry says "the R isomer is more potent in tryptamines", which is the L-tryptamine, right? I seemed to have lost my confidence in such matters.
I will expand on this later. Much too much to think about at the moment.





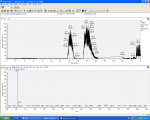
 and eventually ended up with what looked like a thick red tar-like paste. This residue was dissolved in DCM and vinegar, the DCM back extracted with vinegar , vinegar was pooled and basifiied with sodium bicarbonate washed with DCM, and then basified with NaOH. A lower layer of typtamine formed in the bottom of the beaker, the alkaline solution decanted and within 30 minutes the tryptamine had spontaneously crystalized into beautiful off white waxy crystals, air driied overnight and weighing >15g (9.3mmol) for a yield of 94%!!!
and eventually ended up with what looked like a thick red tar-like paste. This residue was dissolved in DCM and vinegar, the DCM back extracted with vinegar , vinegar was pooled and basifiied with sodium bicarbonate washed with DCM, and then basified with NaOH. A lower layer of typtamine formed in the bottom of the beaker, the alkaline solution decanted and within 30 minutes the tryptamine had spontaneously crystalized into beautiful off white waxy crystals, air driied overnight and weighing >15g (9.3mmol) for a yield of 94%!!!