Hey guys, a couple of you may know, epinephrine (adrenaline) is a compound that interests me greatly. After doing some reading, I've found a couple of doable syntheses. For the record, doable is a broad term.
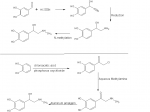
Synthesis A
protocatechualdehyde (3,4-hydroxylbenzaldehyde) is reacted with hydrogen cyanide to produce the cyanhydrin. This is then reduced to the amine. Unfortunately, the only ways to reduce nitriles involve either hydrogenation with palladium or lithium aluminum hydride. The resulting compound is the methylated. This is where I'm just not sure. I don't know if the methylating agent will prefer to attack the nitrogen or not; otherwise it will just methylate the hydroxyl groups on the other side. All in all, this route is pretty useless to us home chemists, as every step involves a hard to obtain material (or very dangerous material)
Synthesis B
Catechol is reacted with monochloroacetic acid in the presence of POCl3. As far as I can tell, the phosphorus oxychloride is only used as a catalyst, but the text did not specify very well. Perhaps thionyl chloride could be substituted as it's nearly impossible to get any phosphorus compounds other than phosphates. The resulting compound is reacted with aqueous methylamine, at which point the corresponding compound will precipitate. This is then simply reduced via an aluminum amalgam reduction.
Synthesis B seems quite feasible to me. Chloroacetic acid can be prepared with acetic acid and chlorine, and I'm sure sulfur can be used instead of phosphorus in the reaction. If thionyl chloride can be used, that can be made in the lab (however with some difficulty). Everything else is very easy for the moderately equipped home laboratory. Perhaps we can find a substitute for the phosphorus oxychloride and/or thionyl chloride all together?
Synthesis A
protocatechualdehyde (3,4-hydroxylbenzaldehyde) is reacted with hydrogen cyanide to produce the cyanhydrin. This is then reduced to the amine. Unfortunately, the only ways to reduce nitriles involve either hydrogenation with palladium or lithium aluminum hydride. The resulting compound is the methylated. This is where I'm just not sure. I don't know if the methylating agent will prefer to attack the nitrogen or not; otherwise it will just methylate the hydroxyl groups on the other side. All in all, this route is pretty useless to us home chemists, as every step involves a hard to obtain material (or very dangerous material)
Synthesis B
Catechol is reacted with monochloroacetic acid in the presence of POCl3. As far as I can tell, the phosphorus oxychloride is only used as a catalyst, but the text did not specify very well. Perhaps thionyl chloride could be substituted as it's nearly impossible to get any phosphorus compounds other than phosphates. The resulting compound is reacted with aqueous methylamine, at which point the corresponding compound will precipitate. This is then simply reduced via an aluminum amalgam reduction.
Synthesis B seems quite feasible to me. Chloroacetic acid can be prepared with acetic acid and chlorine, and I'm sure sulfur can be used instead of phosphorus in the reaction. If thionyl chloride can be used, that can be made in the lab (however with some difficulty). Everything else is very easy for the moderately equipped home laboratory. Perhaps we can find a substitute for the phosphorus oxychloride and/or thionyl chloride all together?