Asymmetric Phase-Transfer Catalysis
Scheme 61 from:
h**p://isites.harvard.edu/fs/docs/icb.topic208898.files/Wed_Oct_3/Phase_Transfer_Catalysis.pdf
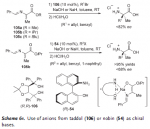
95% yield reported, not that it matters (these are amino acids after all). Notice also the gift of hydrolysis as a side effect. This makes the synthesis for basic unsubstituted amphetamine three steps through either of two routes followed by the usual work up. Common aryl substitutions; hydroxy, methoxy, methylenedioxy, etc, should not interfere. No listed precursers, no listed intermediates, easily handled reagents with proper respect for methylation reagents (Route A), excellent enantioselectivity or racemic mixture depending on the PTC chosen.
Route A: Phenylalanine and MeBr (or MeI, and likely other methyl halides)
Route B: Alanine and benzyl bromide (I don’t know if benzyl chloride would work)
1. Formation of Schiff Base (benzaldehyde, or whatever you like - vanillin?)
2. Asymmetric PTC Alkylation at alpha carbon to nitrogen
3. Decarboxylation
With regard to the PTC, NOBIN is used but I don’t see why BINOL wouldn’t work for a racemic mixture. For experimental details, follow the reference trail.
I would highly suggest going the extra mile for NOBIN however, if you are using a 1,3-benzodioxole varietal and plan to mono-N-methylate. The reason is that it has been shown that the (S) isomer of the primary amine with this moiety is similar in effect to the mono-N-methylated version. It has been suggested that steric effects may be at play with the latter, preventing it from tickling of the 5HT2a receptor so found to be responsible for the psychedelic effects of the (R) isomer (as found in the virtually ubiquitous racemic product available through many older routes).
h**p://www.ncbi.nlm.nih.gov/pubmed/7824160
One could get there through Tyrosine via a couple of extra steps bringing the total to 5 which is left as an exercise for the reader.
I suppose one would have to weigh their options – either get the right PTC to produce an (S) isomer, or proceed with mono-N-methylation. If you choose the later, I suggest reading a post by Klute on Chemical Forums if I recall correctly for a very nice OTC method through the schiff base (coincidentally).
As a side note, it has been reported frequently that the effects of Adderall and Vyvanse (the amphetamine / lysine condensed prodrug) are somewhat different with respect to mood and intensity. There may be something more sophisticated happening with the prodrug than simple enzymatic cleavage of the lysine molecule. I wonder what the effects a similar prodrug with the methylenedioxy group may produce aside from a longer experience? Food for thought.
Amino acids are good. @Sedit, thanks for bringing me here to share this.
Lastly, I propose a toast to Hugo Schiff, who contributed greatly to the world in which we live. From the people of the future, with much respect and love, may you rest in peace. Everyone pour a drink (I’m sober these days, so I’ll do it with a glass of sweet tea) – all together - “Arriba, abajo, al centro y pa’dentro!”
Scheme 61 from:
h**p://isites.harvard.edu/fs/docs/icb.topic208898.files/Wed_Oct_3/Phase_Transfer_Catalysis.pdf
95% yield reported, not that it matters (these are amino acids after all). Notice also the gift of hydrolysis as a side effect. This makes the synthesis for basic unsubstituted amphetamine three steps through either of two routes followed by the usual work up. Common aryl substitutions; hydroxy, methoxy, methylenedioxy, etc, should not interfere. No listed precursers, no listed intermediates, easily handled reagents with proper respect for methylation reagents (Route A), excellent enantioselectivity or racemic mixture depending on the PTC chosen.
Route A: Phenylalanine and MeBr (or MeI, and likely other methyl halides)
Route B: Alanine and benzyl bromide (I don’t know if benzyl chloride would work)
1. Formation of Schiff Base (benzaldehyde, or whatever you like - vanillin?)
2. Asymmetric PTC Alkylation at alpha carbon to nitrogen
3. Decarboxylation
With regard to the PTC, NOBIN is used but I don’t see why BINOL wouldn’t work for a racemic mixture. For experimental details, follow the reference trail.
I would highly suggest going the extra mile for NOBIN however, if you are using a 1,3-benzodioxole varietal and plan to mono-N-methylate. The reason is that it has been shown that the (S) isomer of the primary amine with this moiety is similar in effect to the mono-N-methylated version. It has been suggested that steric effects may be at play with the latter, preventing it from tickling of the 5HT2a receptor so found to be responsible for the psychedelic effects of the (R) isomer (as found in the virtually ubiquitous racemic product available through many older routes).
h**p://www.ncbi.nlm.nih.gov/pubmed/7824160
One could get there through Tyrosine via a couple of extra steps bringing the total to 5 which is left as an exercise for the reader.
I suppose one would have to weigh their options – either get the right PTC to produce an (S) isomer, or proceed with mono-N-methylation. If you choose the later, I suggest reading a post by Klute on Chemical Forums if I recall correctly for a very nice OTC method through the schiff base (coincidentally).
As a side note, it has been reported frequently that the effects of Adderall and Vyvanse (the amphetamine / lysine condensed prodrug) are somewhat different with respect to mood and intensity. There may be something more sophisticated happening with the prodrug than simple enzymatic cleavage of the lysine molecule. I wonder what the effects a similar prodrug with the methylenedioxy group may produce aside from a longer experience? Food for thought.
Amino acids are good. @Sedit, thanks for bringing me here to share this.
Lastly, I propose a toast to Hugo Schiff, who contributed greatly to the world in which we live. From the people of the future, with much respect and love, may you rest in peace. Everyone pour a drink (I’m sober these days, so I’ll do it with a glass of sweet tea) – all together - “Arriba, abajo, al centro y pa’dentro!”