The Dimsyl ion is a very useful super base used in organic synthesis to affect a wide variety of reactions. It can be synthesized in good yeilds thru the addition of a strong base such as an alkali hydride(NaH, KH) or alkali amide(NaNH2). Newer Methods exist that facilitate the generation of the Dimsyl ion as well that include the electrolysis of a soluble alkali salt in DMSO[1]. As it stands this appears to be one of the safest and most readily avalible means of generating the deprotonated form of DMSO in that it does not require the use of dangerous hydrides and amides or the work of Elemental alkali metals.
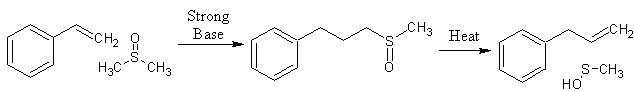
One of the primary uses of the Dimsyl ion which causes me to write this thread is its ability to synthesis Allylbenzene in high yeilds thru the condensation of Styrene with Dimsyl ion and pyrolysis of the resulting intermediate.[2] Something I recently found intriguing is the fact that the addition of Alkali hydroxides is able to form the NaDMSO as well although the concentration is quite low[4]. As such I wounder if the above reaction will be able to proceed to the right by using up the Dimsyl ions as it forms keeping the concentration in the lower end ranges while still allowing the formation the sulfoxide in sufficient yeilds. There are good odds that since H2O is a product here that the addition of a Molecular sieve may be needed to achieve the desired results. If experimentation proves this to be true then all one will need is to mix Styrene and DMSO at room temperature with KOH and allow a long stir at lower temperatures. This can then be followed by a steam distill to effect pyrolysis and liberate the resulting Allylbenzene. If higher temperatures where used to speed up the reaction you would be left with condensation products of Allylbenzene since the resulting sulfoxide degrades with relative ease.
As a methylating reagent:
One can see from this that the Dimsyl ion is effective in the methylation of Carbon-carbon Pi bonds in that it adds the sulfoxide followed with elimination thru refluxing the mixture following the initial reaction.

This also seems like it could provide an effective means for the methylation of ?-nitro styrenes as long as the methylation proceeds at the ?-carbon as well. This means that for once substituted Benzaldahydes can possible be condensed with Nitromethane followed with this treatment of the Dimsyl ion to yeild the corrisponding Nitro compound which would be followed with a reduction to amphetamine.
To facilitate ester Cleavage and conversion to Methyl ketones
There is also another useful little tidbit of information a freind has kindly provided me with which shows one very important use of the Dimsyl ion in organic synthesis. What is interesting about this is its ability to add to Esters followed by a reduction with Al/Hg to yeild the given methyl Ketone[5]. One can envision with little effort the esterfication of Phenylacetic acid followed with treatment with NaDMSO and further reduction to yeild Phenylacetone. This eliminates the need for toxic lead but introduces the use of toxic Mercury so unless it is found that other methods of reduction can be used then I am not sure this is a viable option. However once the reaction mixture already contains Hg I can not help but wounder if the following reduction to an amine could be performed in a single pot reaction.

Conclusion:
As you can see from all of this the formation of the Dimsyl ion is critical in some very useful organic processes. There are quite a few means of synthesis but as it stands the electrochemical synthesis appears to be the most valid and as an added bonus the reference provided shows a means of synthesizing Grignard reagents without the hassles normaly associated with them. Much thanks goes out to our member shroomedalice for bringing this reaction to light for me sometime back and staying on top of it enough to find the electrochemical synthesis that cataylised this threads formation. I would very much like some feedback from our member base on the various uses and generation of the Dimsyl ions.
References:
[1]Versatile electrochemically based preparation of unusual Grignard reagents containing electrophilic substituents
Philippe Dauban, Robert H. Dodd
Tetrahedron Letters Volume 39, Issue 32, 6 August 1998, Pages 5739-5742
[2]The Addition of Dimethyl Sulfoxide Anion to Olefins and the Pyrolysis of Sulfoxides
Cheves Walling, Laszlo Bollyky
J. Org. Chem.,1964, 29 (9), pp 2699–2701
[3]Thermolysis of Alkyl Sulfoxides and Derivatives: A Comparison of Experiment and Theory
Jerry W. Cubbage, Yushen Guo, Ryan D. McCulla, and William S. Jenks
J. Org. Chem. 2001, 66 (26), pp 8722–8736
[4]Basicity of saturated solutions of alkali metal hydroxides in dimethylsulfoxide
B. A. Trofimov1, A. M. Vasil''tsov1 and S. V. Amosova1
Russian Chemical BulletinVolume 35, Number 4 / April, 1986, pg.682-686
[5]Swenton, J. S; Anderson, D. K; Jackson, D. K; Narasimhan, L (1981). "1,4-Dipole-metalated quinone strategy to (±)-4-demethoxydaunomycinone and (±)-daunomycinone. Annelation of benzocyclobutenedione monoketals with lithioquinone bisketals". J. Org. Chem. 46: 4825. doi:10.1021/jo00337a002.
One of the primary uses of the Dimsyl ion which causes me to write this thread is its ability to synthesis Allylbenzene in high yeilds thru the condensation of Styrene with Dimsyl ion and pyrolysis of the resulting intermediate.[2] Something I recently found intriguing is the fact that the addition of Alkali hydroxides is able to form the NaDMSO as well although the concentration is quite low[4]. As such I wounder if the above reaction will be able to proceed to the right by using up the Dimsyl ions as it forms keeping the concentration in the lower end ranges while still allowing the formation the sulfoxide in sufficient yeilds. There are good odds that since H2O is a product here that the addition of a Molecular sieve may be needed to achieve the desired results. If experimentation proves this to be true then all one will need is to mix Styrene and DMSO at room temperature with KOH and allow a long stir at lower temperatures. This can then be followed by a steam distill to effect pyrolysis and liberate the resulting Allylbenzene. If higher temperatures where used to speed up the reaction you would be left with condensation products of Allylbenzene since the resulting sulfoxide degrades with relative ease.
As a methylating reagent:
One can see from this that the Dimsyl ion is effective in the methylation of Carbon-carbon Pi bonds in that it adds the sulfoxide followed with elimination thru refluxing the mixture following the initial reaction.

Quote
The dimsyl ion also adds to carbon-carbon double bonds, and if the mixture is heated for several hours, the initial adduct eliminates methanesulfenic acid. The overall result is methylation and with compounds such as quinoline or isoquinoline, yields are nearly quantitative [Russell, G. A.; Weiner, S. A., J. Org. Chem. 31, 248-251 (1966)] .Source
This also seems like it could provide an effective means for the methylation of ?-nitro styrenes as long as the methylation proceeds at the ?-carbon as well. This means that for once substituted Benzaldahydes can possible be condensed with Nitromethane followed with this treatment of the Dimsyl ion to yeild the corrisponding Nitro compound which would be followed with a reduction to amphetamine.
To facilitate ester Cleavage and conversion to Methyl ketones
There is also another useful little tidbit of information a freind has kindly provided me with which shows one very important use of the Dimsyl ion in organic synthesis. What is interesting about this is its ability to add to Esters followed by a reduction with Al/Hg to yeild the given methyl Ketone[5]. One can envision with little effort the esterfication of Phenylacetic acid followed with treatment with NaDMSO and further reduction to yeild Phenylacetone. This eliminates the need for toxic lead but introduces the use of toxic Mercury so unless it is found that other methods of reduction can be used then I am not sure this is a viable option. However once the reaction mixture already contains Hg I can not help but wounder if the following reduction to an amine could be performed in a single pot reaction.

Conclusion:
As you can see from all of this the formation of the Dimsyl ion is critical in some very useful organic processes. There are quite a few means of synthesis but as it stands the electrochemical synthesis appears to be the most valid and as an added bonus the reference provided shows a means of synthesizing Grignard reagents without the hassles normaly associated with them. Much thanks goes out to our member shroomedalice for bringing this reaction to light for me sometime back and staying on top of it enough to find the electrochemical synthesis that cataylised this threads formation. I would very much like some feedback from our member base on the various uses and generation of the Dimsyl ions.
References:
[1]Versatile electrochemically based preparation of unusual Grignard reagents containing electrophilic substituents
Philippe Dauban, Robert H. Dodd
Tetrahedron Letters Volume 39, Issue 32, 6 August 1998, Pages 5739-5742
[2]The Addition of Dimethyl Sulfoxide Anion to Olefins and the Pyrolysis of Sulfoxides
Cheves Walling, Laszlo Bollyky
J. Org. Chem.,1964, 29 (9), pp 2699–2701
[3]Thermolysis of Alkyl Sulfoxides and Derivatives: A Comparison of Experiment and Theory
Jerry W. Cubbage, Yushen Guo, Ryan D. McCulla, and William S. Jenks
J. Org. Chem. 2001, 66 (26), pp 8722–8736
[4]Basicity of saturated solutions of alkali metal hydroxides in dimethylsulfoxide
B. A. Trofimov1, A. M. Vasil''tsov1 and S. V. Amosova1
Russian Chemical BulletinVolume 35, Number 4 / April, 1986, pg.682-686
[5]Swenton, J. S; Anderson, D. K; Jackson, D. K; Narasimhan, L (1981). "1,4-Dipole-metalated quinone strategy to (±)-4-demethoxydaunomycinone and (±)-daunomycinone. Annelation of benzocyclobutenedione monoketals with lithioquinone bisketals". J. Org. Chem. 46: 4825. doi:10.1021/jo00337a002.





 .
. 