Im pretty sure you guys heard of it but I was wondering if anybody has actually tried it. It was reported in this Japanese article that an Oxazolidine intermediate is formed and further hydrolysis is required in order to maximize yield of amino-alcohol. Refluxing the final mixture with a mixture of acetic acid, benzene and methanol seems to be used but it isnt clear.They report a yield of 47 % racemic ephedrine using N-methylalanine. Not bad at all. The interpretation of the authors has received some criticism though.
Question is has anybody tried it in order to obtain greater yields of racemic PPA ?
Heres a rough translation of the experimental protocol and discussion:
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Amino acid is the useful synthetic raw materials as a cheap asymmetrical source. But, after for solubilizing to the control and the organic solvent of the side reaction, protecting amino acid, the fact that it does the conversion reaction was common sense. We discovered character and the reaction whose amino acid is new the unprotected amino acid which, is almost not examined so far as the synthetic raw materials by using, shortly showed the fact that also synthesis of natural ones with the distance which does not need the distance of 1 and protection and deviation from protection is possible. Because 2 this time we the carbon which accompanies the decarboxylation of unprotected amino acid - developed the practical synthesis method of the amino alcohol which utilizes the carbon connection formation reaction, it reports.
As for Akabori and the like, it is low the N-[mechiru] alanine (1) and in pyridine heating benzaldehyde it reported that by (2) in 1942, yield (16%) you can obtain the ephedrine (3) with one distance (Scheme 1). As for 3 these reactions accompanying the decarboxylation in on the asymmetric carbon of amino acid, because the unique reaction type that [A] is, carbon - it causes carbon connection, after that, with the tall tree and Emoto etc from 1951 extending through 1961 in detail inspection Attacking/questioning it was done, but it did not succeed in the improvement of yield. As for 4 these reactions, because from unprotected amino acid you can obtain the amino alcohol which is included mainly as a basic framework of the medical supply with one distance, the possibility of becoming the useful synthesis reaction is concealed
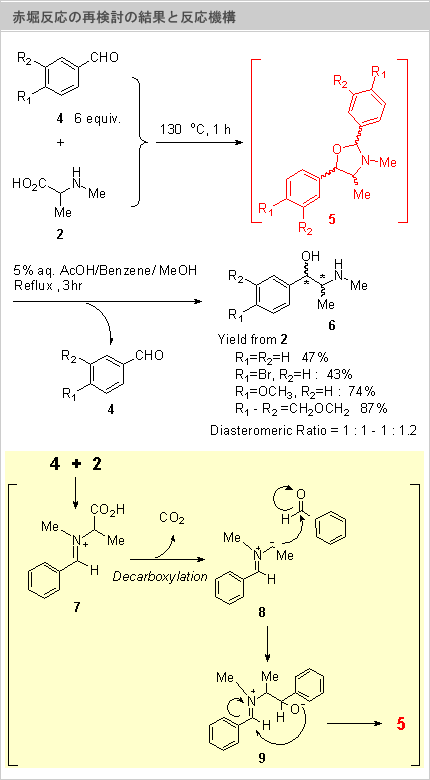
We from the viewpoint of the reaction of unprotected amino acid reexamination did to this reaction with interest, (Scheme 2). The N-[mechiru] alanine (1) and benzaldehyde (4, R=H) with the non solvent 130? and 1hr when it heats, the ephedrine (6, R=H) it was obtained same as Akabori and the like report low at only yield, but when the reaction blend is scrutinized, the Oxazolidine body (5), it became clear from the measurement of NMR to have formed. This chemical compound (5), being unstable, from the fact that isolation is difficult, when adding water it disassembled the reaction liquid directly with the AcOH aqueous solution, improved yield substantially and could obtain 6 at 48% yield as a blend of diastereoisomer. Furthermore this reaction, when it goes with the benzaldehyde which possesses the various substituents, the yield where the benzaldehyde which possesses the electron donating group is better was given, maximum of 87% yield improved (Table and Run 6).The Oxazolidine body (5) formation the Schiff base (7) has suggested formation strongly as a intermediate field. Namely, the decarboxylation happens the Schiff base (7) formation as a trigger, Zwitter Ion 8 occurs, continuously, after the reaction of the aldol type of benzaldehyde, the cyclization reaction inside the molecule of 9 where it forms happens, 5 forming is thought, (Scheme 3). In announcement, the amino acid other than the N-[mechiru] alanine is used when and, it is the schedule which also examination of the solvent and the result etc when the optically active substance is used report together
------------------------------------------------------------------------------------------------------------------
We, from the viewpoint of development of the synthesis reaction of the unprotected amino acid which examines so far, observed to this reaction and reexamination did.
N-Methylalanine (2) with 6 equivalent aromatic aldehydes (4) with the non solvent 130? and 1hr when it heats, same as Akabori and the like report, from water layer, the ephedrine was obtained low at only yield. But, when organic layer is scrutinized the [okisazorijin] body (5) it was found that it has formed in large quantities. This without being isolated when adding water it disassembled with the 5%AcOH aqueous solution, the amino alcoholic body (6) diastereoisomer almost 1: It was obtained at 48% yield, as 1 blends (the right figure). Namely, with Akabori and the like experiment, because the [okisazorijin] body (5) the fact that it has formed, is overlooked in organic layer, it is thought as the thing whose yield is bad. Furthermore, this reaction when it goes with various substitution benzaldehyde, about those where the electron donating group is attached yield improved, was obtained at maximum of 87% yield.
In addition, the [okisazorijin] body (5) formation gives very regarding reaction mechanism important information. Namely, amino acid (2) with aldehyde (4), in minium salt (7) it forms, that becomes the trigger and the decarboxylation happens, Zwitter Ion 8 occurs. The aldehyde of the monad (4) reacting with this 8 already, 9 where it occurs cyclizing, it is thought that the [okisazorijin] body (5) it formed. This [okizazorijin] body (5), being relatively unstable, when it heats with the acetic acid aqueous solution, hydrolysis happens, gives the amino alcoholic body.
I feel that the original reaction which has been buried to this, Japan, has concealed big possibility not only being connected to the development of the organic chemistry where the amino acid which you are proud in the world 'as the Akabori reaction' is new, as a practical synthesis method of the medical supply.
http://www.mnc.toho-u.ac.jp/v-lab/yuuki/amino/amino-5-1.html
Question is has anybody tried it in order to obtain greater yields of racemic PPA ?
Heres a rough translation of the experimental protocol and discussion:
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Amino acid is the useful synthetic raw materials as a cheap asymmetrical source. But, after for solubilizing to the control and the organic solvent of the side reaction, protecting amino acid, the fact that it does the conversion reaction was common sense. We discovered character and the reaction whose amino acid is new the unprotected amino acid which, is almost not examined so far as the synthetic raw materials by using, shortly showed the fact that also synthesis of natural ones with the distance which does not need the distance of 1 and protection and deviation from protection is possible. Because 2 this time we the carbon which accompanies the decarboxylation of unprotected amino acid - developed the practical synthesis method of the amino alcohol which utilizes the carbon connection formation reaction, it reports.
As for Akabori and the like, it is low the N-[mechiru] alanine (1) and in pyridine heating benzaldehyde it reported that by (2) in 1942, yield (16%) you can obtain the ephedrine (3) with one distance (Scheme 1). As for 3 these reactions accompanying the decarboxylation in on the asymmetric carbon of amino acid, because the unique reaction type that [A] is, carbon - it causes carbon connection, after that, with the tall tree and Emoto etc from 1951 extending through 1961 in detail inspection Attacking/questioning it was done, but it did not succeed in the improvement of yield. As for 4 these reactions, because from unprotected amino acid you can obtain the amino alcohol which is included mainly as a basic framework of the medical supply with one distance, the possibility of becoming the useful synthesis reaction is concealed
We from the viewpoint of the reaction of unprotected amino acid reexamination did to this reaction with interest, (Scheme 2). The N-[mechiru] alanine (1) and benzaldehyde (4, R=H) with the non solvent 130? and 1hr when it heats, the ephedrine (6, R=H) it was obtained same as Akabori and the like report low at only yield, but when the reaction blend is scrutinized, the Oxazolidine body (5), it became clear from the measurement of NMR to have formed. This chemical compound (5), being unstable, from the fact that isolation is difficult, when adding water it disassembled the reaction liquid directly with the AcOH aqueous solution, improved yield substantially and could obtain 6 at 48% yield as a blend of diastereoisomer. Furthermore this reaction, when it goes with the benzaldehyde which possesses the various substituents, the yield where the benzaldehyde which possesses the electron donating group is better was given, maximum of 87% yield improved (Table and Run 6).The Oxazolidine body (5) formation the Schiff base (7) has suggested formation strongly as a intermediate field. Namely, the decarboxylation happens the Schiff base (7) formation as a trigger, Zwitter Ion 8 occurs, continuously, after the reaction of the aldol type of benzaldehyde, the cyclization reaction inside the molecule of 9 where it forms happens, 5 forming is thought, (Scheme 3). In announcement, the amino acid other than the N-[mechiru] alanine is used when and, it is the schedule which also examination of the solvent and the result etc when the optically active substance is used report together
------------------------------------------------------------------------------------------------------------------
We, from the viewpoint of development of the synthesis reaction of the unprotected amino acid which examines so far, observed to this reaction and reexamination did.
N-Methylalanine (2) with 6 equivalent aromatic aldehydes (4) with the non solvent 130? and 1hr when it heats, same as Akabori and the like report, from water layer, the ephedrine was obtained low at only yield. But, when organic layer is scrutinized the [okisazorijin] body (5) it was found that it has formed in large quantities. This without being isolated when adding water it disassembled with the 5%AcOH aqueous solution, the amino alcoholic body (6) diastereoisomer almost 1: It was obtained at 48% yield, as 1 blends (the right figure). Namely, with Akabori and the like experiment, because the [okisazorijin] body (5) the fact that it has formed, is overlooked in organic layer, it is thought as the thing whose yield is bad. Furthermore, this reaction when it goes with various substitution benzaldehyde, about those where the electron donating group is attached yield improved, was obtained at maximum of 87% yield.
In addition, the [okisazorijin] body (5) formation gives very regarding reaction mechanism important information. Namely, amino acid (2) with aldehyde (4), in minium salt (7) it forms, that becomes the trigger and the decarboxylation happens, Zwitter Ion 8 occurs. The aldehyde of the monad (4) reacting with this 8 already, 9 where it occurs cyclizing, it is thought that the [okisazorijin] body (5) it formed. This [okizazorijin] body (5), being relatively unstable, when it heats with the acetic acid aqueous solution, hydrolysis happens, gives the amino alcoholic body.
I feel that the original reaction which has been buried to this, Japan, has concealed big possibility not only being connected to the development of the organic chemistry where the amino acid which you are proud in the world 'as the Akabori reaction' is new, as a practical synthesis method of the medical supply.

http://www.mnc.toho-u.ac.jp/v-lab/yuuki/amino/amino-5-1.html




 ) are irrelevant to what QD is asking... With unmethylated alanine apparently dimers and other byproducts are the problem, which significantly decrease yield. N-Methylalanine is going to be a fucking whole lot easier to make if people just look at how alanine is synthesized - either a-Halopropionic acid is used to N-alkylate methylamine in ethanol or you could do a reductive amination of pyruvic acid (dry distillation of tartaric acid would be an obvious route for small scale synthesis) with methylamine and reduction of the imine. In either case, you'd probably be able to save yourself the resolution as the product, if the azlactone does in fact form, will be racemic regardless of which isomer of alanine you start with...
) are irrelevant to what QD is asking... With unmethylated alanine apparently dimers and other byproducts are the problem, which significantly decrease yield. N-Methylalanine is going to be a fucking whole lot easier to make if people just look at how alanine is synthesized - either a-Halopropionic acid is used to N-alkylate methylamine in ethanol or you could do a reductive amination of pyruvic acid (dry distillation of tartaric acid would be an obvious route for small scale synthesis) with methylamine and reduction of the imine. In either case, you'd probably be able to save yourself the resolution as the product, if the azlactone does in fact form, will be racemic regardless of which isomer of alanine you start with...
