When you boil the black permangenate solution it will turn white. You could always add H2O2 like done with cleaning H2SO4 to clear it up as that will not effect the MnSO4 as nitrate ions could later down the like. That should work with dissolved organics and such but if there is a large large amount that needs filtered go with glass wool because paper will disintegrate just leaving you with more organics to deal with.
Sedit
- Global Moderator
- Foundress Queen





- Posts: 2,099
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
If only I had H2O2 in decent quantities at the moment... tsk tsk
I'll probably just boil it down now that I've got my fume hood set up. I won't have to worry about H2SO4 vapor or Mn compounds kicked into the air so that is good. I just need to get a stove now.
I'll probably just boil it down now that I've got my fume hood set up. I won't have to worry about H2SO4 vapor or Mn compounds kicked into the air so that is good. I just need to get a stove now.
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
Just as a note for me I'm posting this:
http://www.patentstorm.us/patents/4146582/fulltext.html
http://www.patentstorm.us/patents/4146582/fulltext.html
Quote
EXAMPLE 5
Benzaldehyde
Ferrous-Copper Catalyst: Toluene (7.6 g.), water (35 ml.), ferrous sulphate (0.110 g.) heptahydrate, cupric acetate (0.072 g.) and methanol (8 ml.) are placed in a 250 ml. reactor.
Sodium persulphate (47.05 g.) in an aqueous-methanol solution of sodium persulphate is added slowly to the mixture which is maintained at 70° C., in an atmosphere of nitrogen and under agitation.
The organic phase is separated after two hours and the aqueous phase is extracted with ethyl ether.
The combined organic phases are distilled to afford 8.29 g. (95% yield) of very pure benzaldehyde (compared against a pure sample).
Sedit
- Global Moderator
- Foundress Queen





- Posts: 2,099
http://www.sciencemadness.org/talk/viewthread.php?tid=2223&page=15
Klute reported mixed success but it seems workable. The large amount of Sodium Persulfate is a put off to me honestly. For 70 grams of toluene you need 470.5 grams of Persulfate just to put it into perspective. Read what Klute posted as he talked of a nasty runaway if your not careful.
Klute reported mixed success but it seems workable. The large amount of Sodium Persulfate is a put off to me honestly. For 70 grams of toluene you need 470.5 grams of Persulfate just to put it into perspective. Read what Klute posted as he talked of a nasty runaway if your not careful.
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
Yeah, but I kind of hate the Mn Persulfate stuff as well. I may just order a few pounds of Mn sulfate however... but I can't find a quantity/price that makes me happy.
NH4 Persulfate can be made from ammonium sulfate, which I already have like 40lbs of it, so I have way more then I need to begin with. I just like that patent though, thanks for the link.
NH4 Persulfate can be made from ammonium sulfate, which I already have like 40lbs of it, so I have way more then I need to begin with. I just like that patent though, thanks for the link.
Sedit
- Global Moderator
- Foundress Queen





- Posts: 2,099
My supplyer for ceramic materials sells MnO2 for $1.78 a pound. Thats cheep enough to make it worth it to make the sulfate your self.
GL with the Na persulfate route I have done alot of thinking about that one myself before I got the Mnpersulfate working.
GL with the Na persulfate route I have done alot of thinking about that one myself before I got the Mnpersulfate working.
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
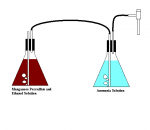
This is my little attempt at making acetaldehyde..
well, in the end, the ammonia-acetaldehyde "hexamine" analogue
The first picture is that of a 1 gallon glass jug with a fish heater in it set to make it ~50*C full of my old Mn Persulfate solution and some ethanol.
The second picture is that of the 1 gallon jug with the Mn sulfate and sulfuric acid.
I saw bubbles of gas coming off of it while it was turning colors, and I did smell
green-apple like smells, so i guess that was acetaldehyde. The lid didn't seal thanks to the cord, but I pulled a light vac on it via an aspirator, and sucked in air through the leak, while I bubbled the gas formed through an alcohol solution, and then an ammonia solution, later they were combined and made sure excess ammonia was present. I am going to allow the solution to evaporate and hopefully see some nice crystals of ammonia-acetaldehyde stuff.
When I acquire a large glass jar (1-2 gallon) and 4lbs of Mn sulfate at ~15 bucks, I am going to set up the electrolysis thingy again, and have it be better then before.
I want everything glass this time, since obviously Mn Persulfate is a pretty good oxidizer. I made a bit of unrecoverable benzoic acid yesterday.
well, in the end, the ammonia-acetaldehyde "hexamine" analogue
The first picture is that of a 1 gallon glass jug with a fish heater in it set to make it ~50*C full of my old Mn Persulfate solution and some ethanol.
The second picture is that of the 1 gallon jug with the Mn sulfate and sulfuric acid.
I saw bubbles of gas coming off of it while it was turning colors, and I did smell
green-apple like smells, so i guess that was acetaldehyde. The lid didn't seal thanks to the cord, but I pulled a light vac on it via an aspirator, and sucked in air through the leak, while I bubbled the gas formed through an alcohol solution, and then an ammonia solution, later they were combined and made sure excess ammonia was present. I am going to allow the solution to evaporate and hopefully see some nice crystals of ammonia-acetaldehyde stuff.
When I acquire a large glass jar (1-2 gallon) and 4lbs of Mn sulfate at ~15 bucks, I am going to set up the electrolysis thingy again, and have it be better then before.
I want everything glass this time, since obviously Mn Persulfate is a pretty good oxidizer. I made a bit of unrecoverable benzoic acid yesterday.
Sedit
- Global Moderator
- Foundress Queen





- Posts: 2,099
Will acetaldahyde work like formaldehyde does? Either way nice work. That's a whole Lotta oxidiser you have there oh the fun I could have with that. I never isolated any of the aldehyde but it surely was there without a doubt.
I pretty much got the workup down packed now for the BnO and ill share in the morning some photos of what I'm using. Sad thing is my oxidiser is contaminated with Cu and now because the coating came off my stirbar Iron. There should be no benzoic acid created and it sounds as though your solution is to weak. Ill help ya trouble shoot it tomorrow for now I'm off to dream land with the smell of Cherry's still on me to give pleasent dreams.
Also BTW if you are getting BnOOH from toluene then theres a good chance I would think that alot of your product now will be acetic acid.
I pretty much got the workup down packed now for the BnO and ill share in the morning some photos of what I'm using. Sad thing is my oxidiser is contaminated with Cu and now because the coating came off my stirbar Iron. There should be no benzoic acid created and it sounds as though your solution is to weak. Ill help ya trouble shoot it tomorrow for now I'm off to dream land with the smell of Cherry's still on me to give pleasent dreams.
Also BTW if you are getting BnOOH from toluene then theres a good chance I would think that alot of your product now will be acetic acid.
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
well, I got it way to hot with the toluene, slightly on purpose, but I never smelt any BnO 
It is a weak solution, sorta. I accidentally added ~2 the sulfuric acid then what was best for the MnSO4, and a lot of the water evaporated so now I know the concentration of nothing.
I plan to do a titration (needed it in its reduced form for this) soon, and then I'll add the proper amounts of H2O and MnSO4 when I get them.
It'll actually produce way more then I ever wanted this project too.. I don't even really need any aldehyde, etc atm, but might as well.
according to rhodium's archive, acetaldehyde reacts with ammonia to form some compound that can release acetaldehyde with the addition of sulfuric acid.
I think when everything is set up and running really well I am going to post a nice write up about the reaction, tips, etc for Mn persulfate oxidation. That way a bunch of the information will be all in one place, instead of all over the board like it is now.

It is a weak solution, sorta. I accidentally added ~2 the sulfuric acid then what was best for the MnSO4, and a lot of the water evaporated so now I know the concentration of nothing.
I plan to do a titration (needed it in its reduced form for this) soon, and then I'll add the proper amounts of H2O and MnSO4 when I get them.
It'll actually produce way more then I ever wanted this project too.. I don't even really need any aldehyde, etc atm, but might as well.
according to rhodium's archive, acetaldehyde reacts with ammonia to form some compound that can release acetaldehyde with the addition of sulfuric acid.
I think when everything is set up and running really well I am going to post a nice write up about the reaction, tips, etc for Mn persulfate oxidation. That way a bunch of the information will be all in one place, instead of all over the board like it is now.
Sedit
- Global Moderator
- Foundress Queen





- Posts: 2,099
The trace amounts of Im assuming toluene and other materials over power the smell of BnO at first. You can tell this by sticking a glass rod in it as its stirring and smelling it. After a few seconds the other smells will be gone leaving you with a pleasent BnO smell instead of the harsh stinging smell of BnO. I have since lost tract of my concentration as well because of steam distilling it out and leaking of the anode and cathode fluids thru the barrier but im not sure they are really important for success just for great yeilds.
My last run was contaminated with Iron and Copper yet even before redistilling it the BnO began to polymerize overnight which indicates a high concentration. I combined my last couple runs and am going to distill when the temperature here drops below 100degrees because its just to damn hot to leave the nice air conditioning right now.
Lemme know how the acetaldehyde works out.
PS I have been running my toluene in the dirrect sun at about 110degrees from the absorbed heat and its going good.
My last run was contaminated with Iron and Copper yet even before redistilling it the BnO began to polymerize overnight which indicates a high concentration. I combined my last couple runs and am going to distill when the temperature here drops below 100degrees because its just to damn hot to leave the nice air conditioning right now.
Lemme know how the acetaldehyde works out.
PS I have been running my toluene in the dirrect sun at about 110degrees from the absorbed heat and its going good.
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
Nice! I thought about using heat from the sun since the solution is black, it would probably heat it up to 50*C since its also blistering hot outside.
I added a mixture of sodium bisulfite and sodium hydrosulfite to the toluene that was left on top, stirred it a bit and added some water. I noticed the solution turned an orange color. I figured that meant something was produced, but I never could smell BnO.
I'm thinking my toluene source has alcohol and other junk in it, so before I do this again I will wash it a bunch with some water and see how that goes.
Also I forgot to mention that I also could smell a small amount of acetic acid after I was done with the acetaldehyde reaction.
I added a mixture of sodium bisulfite and sodium hydrosulfite to the toluene that was left on top, stirred it a bit and added some water. I noticed the solution turned an orange color. I figured that meant something was produced, but I never could smell BnO.
I'm thinking my toluene source has alcohol and other junk in it, so before I do this again I will wash it a bunch with some water and see how that goes.
Also I forgot to mention that I also could smell a small amount of acetic acid after I was done with the acetaldehyde reaction.
Sedit
- Global Moderator
- Foundress Queen





- Posts: 2,099
Vesp I remember you mentioning using H2O2 you clean your persulfate solution of organic... bee careful. Its more then likely due to the Iron sulfate present in my solution creating the fentons reagent but a capful of H2O2 even though it quickly brought the color back to normal heated up something fierce and decomposed the H2O2 foaming like a bitch. It works but take care in doing it.
You said you added the sulfite to the toluene, could you elaborate a bit more.
You said you added the sulfite to the toluene, could you elaborate a bit more.
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
The toluene that I oxidized a little bit, but found it to give no smell I tossed in some of the bisulfite and shook it around, a little bit of water was present in the form of a few drops at the bottom at the time. Nothing happened obviously and after letting it sit for a bit I added water to it. When I added water to it, the water turned bright orange. Nothing ever seemed to precipitate, or do much so I added more water, and then tossed it.
What color would benzaldehyde bisulfite abduct be and how soluble is it in water? I've never played with the stuff and... haven't recently read much about it.
What color would benzaldehyde bisulfite abduct be and how soluble is it in water? I've never played with the stuff and... haven't recently read much about it.
Sedit
- Global Moderator
- Foundress Queen





- Posts: 2,099
I don't think you'll get there that way Vesp. You should have tryed to seperate the water and free the adduct with NaOH to see if you recovered any.
The adduct itself is a white crystaline material. Perhaps you could practice a little bit with acetone. I prefer to practice with Caverone from Dill oil because it simulates the experiance more being an oil. The idea is to have an extreamly saturated Bisulfite solution to keep the solubility of the adduct to a minmum. Stir it for sometime then place it in the freezer for some cottage cheese looking crap.
Heres a few links.
http://www.sciencemadness.org/talk/viewthread.php?tid=7689
http://designer-drugs.com/pte/12.162.180.114/dcd/chemistry/eleusis/bisulfite.html
Len also posted the process in the Clorination of toluene threed.
The adduct itself is a white crystaline material. Perhaps you could practice a little bit with acetone. I prefer to practice with Caverone from Dill oil because it simulates the experiance more being an oil. The idea is to have an extreamly saturated Bisulfite solution to keep the solubility of the adduct to a minmum. Stir it for sometime then place it in the freezer for some cottage cheese looking crap.
Heres a few links.
http://www.sciencemadness.org/talk/viewthread.php?tid=7689
http://designer-drugs.com/pte/12.162.180.114/dcd/chemistry/eleusis/bisulfite.html
Len also posted the process in the Clorination of toluene threed.
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
Well, I was just seeing if the volume changed or if anything happened. I figured it wouldn't work since my bisulfite source contains hydrosulfite and the formed solution bubbles of SO2. Does /sodium dithionite cause problems?
Orange color probably infers condensation products of possible aldehyde/ketones present in the toluene. SO I am going to take whatever happened as good news.
Orange color probably infers condensation products of possible aldehyde/ketones present in the toluene. SO I am going to take whatever happened as good news.
Sedit
- Global Moderator
- Foundress Queen





- Posts: 2,099
I just thought of something vesp if you want to catch your formed acetaldahyde you could pass it into a Bisulfite solution and save the powdered adduct. Then you could free it as needed by dripping NaOH or Sodium carbonate on it. Just a thought.
How did passing it into ammonia work out anyway?
How did passing it into ammonia work out anyway?
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
I haven't checked, it should be evaporated by now though and hopefully I'll see some nice crystals.
It is funny, the glass gallon jug I used, which could have originally been full of apple juice now is the color of apple juice and it smells like apple juice, or rotting/fermenting apples. How very misleading!
This is a picture of how I sorta had it, and will have it next time. I like this because the bubbling air in the Mn persulfate solution will really help kick out the acetaldehyde I think, and prevent it even more from going to acetic acid.
Ammonia is cheaper for me then bisulifte, so I think I will stick with that, assuming I don't have problems with it. Preferably, I'd like to get it into something similiar to paraldehyde, where it has the use of acetaldehyde, but is much easier to deal with.
It is funny, the glass gallon jug I used, which could have originally been full of apple juice now is the color of apple juice and it smells like apple juice, or rotting/fermenting apples. How very misleading!
This is a picture of how I sorta had it, and will have it next time. I like this because the bubbling air in the Mn persulfate solution will really help kick out the acetaldehyde I think, and prevent it even more from going to acetic acid.
Ammonia is cheaper for me then bisulifte, so I think I will stick with that, assuming I don't have problems with it. Preferably, I'd like to get it into something similiar to paraldehyde, where it has the use of acetaldehyde, but is much easier to deal with.
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
This is just something for me to think about later...
naphthalene =HNO3/H2SO4=> 1-nitronaphthalene =HCl/Fe=> 1-aminonaphthalene =reference=> 1-naphthaldehyde (maybe)
.. useful in making JWH-018, probably.
ref: http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?rxntypeid=80&prep=CV5P0139 - formaldoxime, CuSO4, Na2SO4, NaOAc, H2O
naphthalene =HNO3/H2SO4=> 1-nitronaphthalene =HCl/Fe=> 1-aminonaphthalene =reference=> 1-naphthaldehyde (maybe)
.. useful in making JWH-018, probably.
ref: http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?rxntypeid=80&prep=CV5P0139 - formaldoxime, CuSO4, Na2SO4, NaOAc, H2O
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
Well, someone just got 5lbs of MnSO4! 
Now enough about him, today I have titrated my solution of MnSO4, and H2SO4 finally, I used 10ml of the acid stuff, and it took ~91mls of 1 molar solution of sodium bicarbonate to neutralize it.
It was really difficult to tell when it was neutralized using sodium bicarbonate and pH strips as well as some weird berry that gives a pink solution when acidic and a clear-clue solution when going to be basic. Either way, I don't know if I should trust my data. It would mean it is a 4.6 molar solution, I think (I really need to check the math, I did it really fast on a phone calculator)
I also made my 1molar sodium bicarbonate solution, so.. I'm pretty sure I've hit all the places where one can screw up. Hopefully it is right though.
I am now going to attempt to titrate the MnSO4 in the solution. I've used 10mls again, add an excess of sodium bicarbonate again and boiled it a bit. This has got the tan colored MnCO3 to precipitate, I will rinse the solution on top out with some clean water and make sure no more bicarb is dissolved in it. Then I'll make a 1mol solution of oxalic acid (its just what I have that is pure enough for this..) and figure out how much is in there..
I swear though that I had too much sulfuric acid in there at one time, but now it seems as if I don't have enough - wtf? 4.6 molar solution?
However, I am not really concerned with its concentration, the persulfate forms and is stable for months in it.
I just actually wanted to know exactly what the hell I was doing for once
Time to fork out 15 bucks for a 2 gallon glass jar I suppose
Edit: Actually.. I have sodium bisulfate and that should also work to titrate it.

Now enough about him, today I have titrated my solution of MnSO4, and H2SO4 finally, I used 10ml of the acid stuff, and it took ~91mls of 1 molar solution of sodium bicarbonate to neutralize it.
It was really difficult to tell when it was neutralized using sodium bicarbonate and pH strips as well as some weird berry that gives a pink solution when acidic and a clear-clue solution when going to be basic. Either way, I don't know if I should trust my data. It would mean it is a 4.6 molar solution, I think (I really need to check the math, I did it really fast on a phone calculator)
I also made my 1molar sodium bicarbonate solution, so.. I'm pretty sure I've hit all the places where one can screw up. Hopefully it is right though.
I am now going to attempt to titrate the MnSO4 in the solution. I've used 10mls again, add an excess of sodium bicarbonate again and boiled it a bit. This has got the tan colored MnCO3 to precipitate, I will rinse the solution on top out with some clean water and make sure no more bicarb is dissolved in it. Then I'll make a 1mol solution of oxalic acid (its just what I have that is pure enough for this..) and figure out how much is in there..
I swear though that I had too much sulfuric acid in there at one time, but now it seems as if I don't have enough - wtf? 4.6 molar solution?
However, I am not really concerned with its concentration, the persulfate forms and is stable for months in it.
I just actually wanted to know exactly what the hell I was doing for once

Time to fork out 15 bucks for a 2 gallon glass jar I suppose

Edit: Actually.. I have sodium bisulfate and that should also work to titrate it.
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130

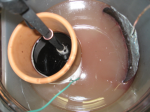
Just some pics..
Never got that graphite electrode to conduct through that 1L terracotta cylinder.. but did conclude that it was the cylinders fault.. so letting it sit over night.. guess so the solutions can connect or do whatever the hell they do to make it conductive..
A small copper wire + the large lead sheet is what gave it that pink color. Makes be wonder if using copper with out a partitioned cell would work well, as long as the copper part had a very low surface area and the Pb plate had a large surface area.
It would eventually reach equilibrium where the Mn persulfate was being destroyed at the same rate it was being produced, but if the copper wire had a small area - the equilibrium would probably be very much in favor of having a bunch of Mn persulfate to only a little MnSO4... So consider that for simplicity.
In total.. this project only cost.. ~35 ish bucks at most