I tracked this down to find out that it had been posted at Science Madness sometime ago but the link was dead. The person that posted the link kindly retrieved the file for me as well. It is about the synthesis of "Super bases" and Proton sponges. Even though I have yet to see one thats as strong as we need there are quite a few that are damn close but are pretty complicated.
Non the lest I will post it for all your reading pleasures. Im sure theres a little something for everyone in this book.
Non the lest I will post it for all your reading pleasures. Im sure theres a little something for everyone in this book.




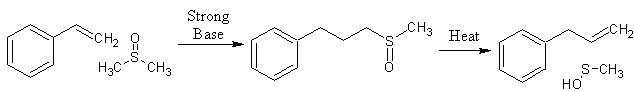


 . I want a bunch of new ones too but this reaction just has not been studied as much as I wish it was, its very very powerful.
. I want a bunch of new ones too but this reaction just has not been studied as much as I wish it was, its very very powerful. . Perhaps this is the result of hinderence due to the bulky nature of the arylamines and I would love to see if any of these reactions worked using Methylamine or some other amine source.
. Perhaps this is the result of hinderence due to the bulky nature of the arylamines and I would love to see if any of these reactions worked using Methylamine or some other amine source.