I regard the Vespiary and Blacklight as the top-tier chemistry forums so I'm cross-posting this here from BL.
Hello Everyone,
I have been researching Endocannabinoids lately and have come to the conclusion that anandamide and its analogs could be extremely useful compounds. I believe of all the analogs studied so far, the three below are the most interesting.
(R)-(+)-Methanandamide
ACEA
ACPA
I can attach structures if requested. ACEA and ACPA don't seem to inhibit FAAH so an inhibitor would probably be needed. Also, ACEA looks like it could be an alkylating agent and could possibly bond to various receptors which is bad news. (R)-(+)-Methanandamide is metabolically stable, and is not hydrolyzed by FAAH.
I'd like this thread to spark discussion on unique Endocannabinoid analogs, especially anandamide. Discussion should include theoretical activity, safety, possible metabolites, etc.
Also, I have scoured forums and any medical articles I could find related to (R)-(+)-Methanandamide and have not found a single human bioassay. Has anyone here heard of any human bioassays on this material? If not why would you think this is? I don't see anything about the compound that screams out toxicity or irreversible damage.
I would love to hear from synapse on this subject. Your novel compound BLK-S-2 is wonderful and I would like to hear from you about the possible toxicity compared to other popular cannabinoids in the JWH series. Hmm, maybe I should address this in the Bioassay thread?
EDIT:
Forgot to add one thing. It looks like ACEA is being offered by a few overseas suppliers which I believe could be extremely dangerous if it is in fact an alkylating agent. If it is being offered by this many suppliers it can only mean that vendors are either using this compound or planning on using this compound in cannabinoid herbal blends. Any insight?
EDIT2:
Last one I promise. Various N-acylethanolamines have been shown to be bioactive. Analogs could prove to be highly potent. I have not studied this much but I believe they interact by temporarily increasing natural levels of anandamide in the body. Any further information would be valuable for all due to the ease of synthesis.
Hello Everyone,
I have been researching Endocannabinoids lately and have come to the conclusion that anandamide and its analogs could be extremely useful compounds. I believe of all the analogs studied so far, the three below are the most interesting.
(R)-(+)-Methanandamide
ACEA
ACPA
I can attach structures if requested. ACEA and ACPA don't seem to inhibit FAAH so an inhibitor would probably be needed. Also, ACEA looks like it could be an alkylating agent and could possibly bond to various receptors which is bad news. (R)-(+)-Methanandamide is metabolically stable, and is not hydrolyzed by FAAH.
I'd like this thread to spark discussion on unique Endocannabinoid analogs, especially anandamide. Discussion should include theoretical activity, safety, possible metabolites, etc.
Also, I have scoured forums and any medical articles I could find related to (R)-(+)-Methanandamide and have not found a single human bioassay. Has anyone here heard of any human bioassays on this material? If not why would you think this is? I don't see anything about the compound that screams out toxicity or irreversible damage.
I would love to hear from synapse on this subject. Your novel compound BLK-S-2 is wonderful and I would like to hear from you about the possible toxicity compared to other popular cannabinoids in the JWH series. Hmm, maybe I should address this in the Bioassay thread?
EDIT:
Forgot to add one thing. It looks like ACEA is being offered by a few overseas suppliers which I believe could be extremely dangerous if it is in fact an alkylating agent. If it is being offered by this many suppliers it can only mean that vendors are either using this compound or planning on using this compound in cannabinoid herbal blends. Any insight?
EDIT2:
Last one I promise. Various N-acylethanolamines have been shown to be bioactive. Analogs could prove to be highly potent. I have not studied this much but I believe they interact by temporarily increasing natural levels of anandamide in the body. Any further information would be valuable for all due to the ease of synthesis.





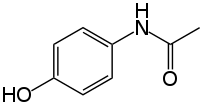


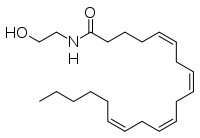

 As long as the inhibitors are irreversible and don't covalently bond they seem relatively safe. Enkidu: Any worries about that Nitrogen Dioxide molecule coming off after ingestion?
As long as the inhibitors are irreversible and don't covalently bond they seem relatively safe. Enkidu: Any worries about that Nitrogen Dioxide molecule coming off after ingestion?