A 1" stirbar is ok with a 2l flask, with a bigger one you must be absolutely sure that your stirplate will pull it through a highly viscous liquid, what is not to expect if you dont have a heavy duty model.
Stirrbar size is a little bit like penis size, or it was at least with everybody claiming to have a bigger one. Whats nonsense of course, as real men in realworld use overhead stirrers for tasks like this.
Once you had one in use you wont go back. Same with a big penis btw. 
/ORG
Can any type of agitator be used for a DIY over head stirrer? Like the type that is placed on a screw driver to mix concrete or does it have to be made of a specific material?
Thanks,
Flight
This now is true for the Al/Hg and for Fe/HCl reactions, it may or may not be true for other reactions! A stainless steel tube or rod can be used as shaft and 3mm PTFE can be cut for blades. Half-moon shaped blades running 0,5cm, not more, above the flasks bottom. in a 2l flask 7 to 8cm wide is enough, stirrer speed wont exceed 400rpm at most anyways.
Some wellknown chinese lab-discounter sells stirrer bearings at less then 20$ and the stirrblades and shafts are rather cheap too - I made most of my own things myself but looking back I would just buy it.
If you want to improvise you need:
- A motor with rpm's regulated
as most practical are known motors from sewing machines. They have the torque and a nice footregulator. I would not worry to much about explosions - good ventilation is a must anyways or you get deadsick from methylamine poisoning. Famous PARR was when I looked the last time still selling their shakers (hydrogen! pressure!...) with cheapo motors - explosion prove costs extra. So lets not get more catholic then the pope here....
- a flexile connection of motor to stirshaft
Thats just a rod of some elastic plastic, something sturdy, the GFK rods for gardening for example. You need just 15 to 20cm. Red rubbertubing to connect this, I show you a picture.
Dont use the infamous vacuum rubber tube connector as shown in many if not most older chemistry books. Say just some of such tubing and thats it! You are heading into disaster with this in a reaction which gets hard to stirr. The reason is that sometimes something tries to block the blade and then the weakest part of the contraption gives way. And thats the tubing. It will twist like a corkscrew and in doing so it will shorten but with an incredible force. It will rip your setup into pieces before you can spell "SHIT!" shattering your flask if you are unlucky.
Using the plastic rod as described the PTFE blade will bend and nothing bad happens, a hiccup nothing more- A rod. SS tube 10x1mm. Take a hammer, flatten one end on 10cm length and drill a hole. SS for the screw to fix the blade which must pivot to get through the neck.
- The blade from PTFE 3mm thick, no other affordable plastic can be used, thats not expensive, though for making two yourself you can buy one in china.
- stirrer bearing to lead the shaft through the neck and stabilize it.
Thats tricky to make, buy it! Or use the suggestion from the post above but you must take two rubber stoppers fixed ass to ass so the earing is long enough to take the load. Also dont inser one glasstube (which will break, as glass doesnt bend, but use two shorter glasstube from top and from bottom and some space between. The glass must be completely in the rubber, no peeking out.
pic 1: flexible connection
pic 2: SS shaft and PTFE blade after some iron/HCl. Oxalic acid cleans it.
/ORG






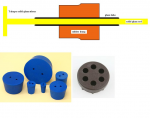



 The end results from the effort applied
The end results from the effort applied 