Someone put me out of my misery...
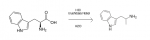
Cinnamic acid yes or no
the MUST be a way here.. i mean look at it ffs.. so close yet so far away
LOL i just checked jons posts from science madness hahaha No wonder you call me jons brother i am exactly the same, maybe jon 10 years ago thats me
haha that is so something that would happen to me
Cinnamic acid yes or no
the MUST be a way here.. i mean look at it ffs.. so close yet so far away
LOL i just checked jons posts from science madness hahaha No wonder you call me jons brother i am exactly the same, maybe jon 10 years ago thats me
Quote
ummm mgso4 does'nt work for drying ammonia amine solutions already tried it.
and why bother passing ammonia water vapor through a column of NaOH?
i can tell you haven't actually done this, because what happens is NaOH cakes up and the back pressure builds and ammonia escapes and you get gassed.
haha that is so something that would happen to me




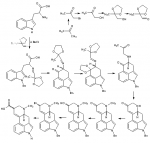
 and the one copy I do have is seriously outdated. I may just see if I can find that old copy I have around the house.
and the one copy I do have is seriously outdated. I may just see if I can find that old copy I have around the house.
