No heat, just sat for a couple days in a test tube rack. Most certainly not your idea situation for producing paraformaldehyde.
Douchermann
- Dominant Queen




- Posts: 397
2bfrank
- Guest
Cool, I'll play around a little, with amounts - what not.. I just cannot find it in any of the camper places where I am. I am also abit spun over purchasing from anywhere other than OTC.... Biding some time on that issue...
thanx again..
thanx again..
Happyman
- Subordinate Wasp



- Posts: 122
The reaction L-PAC+Methylamine=Ephedrine needs a catalyst of some sort (Raney nickel, Pd/C, platinum, ect.)
3 Questions.
Am I correct in assuming that these are necessary to the reaction and not just to speed it up?
What are some alternatives to these catalysts?
And what is "activated aluminium? (Does it have to do with Al/Hg?)
Here is where I see activated aluminum rather than aluminum amalgam.
http://www.wetdreams.ws/forum/mainuploadsfolder/methlab/2005121115044_L-Pac.pdf
3 Questions.
Am I correct in assuming that these are necessary to the reaction and not just to speed it up?
What are some alternatives to these catalysts?
And what is "activated aluminium? (Does it have to do with Al/Hg?)
Here is where I see activated aluminum rather than aluminum amalgam.
http://www.wetdreams.ws/forum/mainuploadsfolder/methlab/2005121115044_L-Pac.pdf
Douchermann
- Dominant Queen




- Posts: 397
@2b - try different walmarts for para. The two most local ones do not have it, but if I go to a superwally world that's about 30 miles south-east from me, they have quite a lot of it.
@Happyman - The catalyst is necessary. Infact, wherever a catalyst is employed, it's pretty much not optional (think degredation of H2O2 with catalyst, formation of acetone peroxide with catalyst, and wacker oxidation with PdCl2). If those metals are being used, you're pretty much stuck with those, or catalysts of that type. Be glad it's not a requirement to use platinum hahaha. All is not lost though, if you're going through the trouble, might as well buy some PdCl2 and make the Pd/C yourself.
As for activated aluminum, read the abstract of this patent for a quick run down.
http://www.freepatentsonline.com/7235226.html
@Happyman - The catalyst is necessary. Infact, wherever a catalyst is employed, it's pretty much not optional (think degredation of H2O2 with catalyst, formation of acetone peroxide with catalyst, and wacker oxidation with PdCl2). If those metals are being used, you're pretty much stuck with those, or catalysts of that type. Be glad it's not a requirement to use platinum hahaha. All is not lost though, if you're going through the trouble, might as well buy some PdCl2 and make the Pd/C yourself.
As for activated aluminum, read the abstract of this patent for a quick run down.
http://www.freepatentsonline.com/7235226.html
2bfrank
- Guest
Yes Im still looking, allthough, those names arent exactly familiar as those names etc.
Also, anyone able to access JOC articles.. I can, but at late, the papers have a biz name associated which could fukmeup, so Cannot do, but here is the title. and it did not delve into named lower primary alkanes, but I dont know if it would be that much of a stretch.. Sort of a surprize..It also compared the traditional, DMF, DMSO AgN02 etc
re: J. Org. Chem. 2004, 69, 6907-6908
The First Conversion of Primary Alkyl
Halides to Nitroalkanes under Aqueous
Medium
Also, anyone able to access JOC articles.. I can, but at late, the papers have a biz name associated which could fukmeup, so Cannot do, but here is the title. and it did not delve into named lower primary alkanes, but I dont know if it would be that much of a stretch.. Sort of a surprize..It also compared the traditional, DMF, DMSO AgN02 etc
re: J. Org. Chem. 2004, 69, 6907-6908
The First Conversion of Primary Alkyl
Halides to Nitroalkanes under Aqueous
Medium
Douchermann
- Dominant Queen




- Posts: 397
Yes Im still looking, allthough, those names arent exactly familiar as those names etc.
If you were referring to the name "super wally world" that's just a name for super walmart my friends and I coined.
rocketman
- Larvae

- Posts: 40
feel free to call it retarded.
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
Look up something called a Grignard Reaction.
I don't think your reactions are possible.
There should be a patent for this drug out there, and I bet it says how to make it.
I don't think your reactions are possible.
There should be a patent for this drug out there, and I bet it says how to make it.
2bfrank
- Guest
Yes Im still looking, allthough, those names arent exactly familiar as those names etc.
If you were referring to the name "super wally world" that's just a name for super walmart my friends and I coined.
lol..Did you think poor 2b was checking out all the local super wally worlds. lol Na, we dont have walmarts full stop....
well actually thats total crap,, poor 2b, contacted 10 good friends.." have you ever heard of a SUPER wally world"" lol's and he got no response.
rocketman
- Larvae

- Posts: 40
Ok, whatever I could never synthesize this anyways. Well theoretically I could maybe, it's not too hard to synthezise diiodomethane and iodomethane, combining which would give 1,2, iodoethane + HI... I could try it when I get the right equipment.
Oh but right it might not work in the first place. Hmm I couldn't find the patent it's an old japanese drug... oh well whatever. One can only dream.
Oh but right it might not work in the first place. Hmm I couldn't find the patent it's an old japanese drug... oh well whatever. One can only dream.
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
Quote
Insert Quote
Ok, whatever I could never synthesize this anyways. Well theoretically I could maybe, it's not too hard to synthezise diiodomethane and iodomethane, combining which would give 1,2, iodoethane + HI... I could try it when I get the right equipment.
Oh but right it might not work in the first place. Hmm I couldn't find the patent it's an old japanese drug... oh well whatever. One can only dream.
Bluelight has a lot of information of lefetamine and its analogues I believe, check there..
Please look up a grignard reaction! How is it you plan to combine diiodomethane with iodomethane? The boiling point of methyl iodide is low, and it is extremely toxic. Why wouldn't iodomethane just react with other iodomethane to form a polymer type substance to begin with?
You'd have the best luck making 1,2-Diiodoethane by reacting HI formed in situation with Ethylene glycol and refluxing/distilling off the 1,2-diiodoethane. again, 1,2-diiodoethane might not even be a practical way to synthesize the drug.
Also, why do you think alkyl iodides are just going to react with benzene how you want them too?
You'll need phenylmagnesium bromide, in anhydrous diethyl ether (a grignard reagent) and it is likely to react with the chloride as well as the iodide on your 1,2-diiodo-2-bromobutane.
You'll end up with a mixture of all sorts of junk.
Although I am no expert in chemistry, I'd like to propose that one method might be to get benzyl magnesium bromide and react that with benzaldehyde. This will form 1,2-diphenyl-ethanol. You could then oxidize the secondary alcohol to a ketone. the ketone could possibly be reductively aminated with dimethylamine to afford the compound you want.
--forming the 1,2-diphenyl-ethanol could be possible with a barbier reaction, the cousin of the grignard reaction.
KMnO4 I believe would have the ability to oxidize this secondary alcohol into a ketone.
rocketman
- Larvae

- Posts: 40
ahh you're right... all those methyl iodides and diiodides would in all likelyhood form chains of mess with each other, same for the dichloromethane with diiodoethane. It would be a big complete polymer mess like you said. I think if one could form 1,2-iodo,1-chloro-propane then they'd be all set but that compound in itself is probably impossible.
That benzyl grignard with benzaldehyde does make sense i guess I'll have to think about that.
That benzyl grignard with benzaldehyde does make sense i guess I'll have to think about that.
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
As I asked you earlier:
They don't react with each other.... as I tried to get you to realize earlier.
Grignards are extremely hard to keep dry, hence I also suggested the barbier reaction, which I strongly recommenced you look up.
There is not a shake and bake method to lefetamine so get over that idea..
If I were you, I'd work on learning some more basic chemistry before going into organic synthesis. Maybe make a few salts that can be useful later on in organic chemistry such as potassium nitrite and stuff like that.
Quote
How is it you plan to combine diiodomethane with iodomethane? The boiling point of methyl iodide is low, and it is extremely toxic. Why wouldn't iodomethane just react with other iodomethane to form a polymer type substance to begin with?
They don't react with each other.... as I tried to get you to realize earlier.
Grignards are extremely hard to keep dry, hence I also suggested the barbier reaction, which I strongly recommenced you look up.
There is not a shake and bake method to lefetamine so get over that idea..
If I were you, I'd work on learning some more basic chemistry before going into organic synthesis. Maybe make a few salts that can be useful later on in organic chemistry such as potassium nitrite and stuff like that.
rocketman
- Larvae

- Posts: 40
Alright, whatever this is far too complicated for me... I won't be able to do past 1 or 2 steps without it all messing up.
And also that sketch I did was wrong, that 3-carboned iodine chloro whatever should have only had 2 carbons.
But don't worry you're right Benzoin is going to be incredibly easier.... Just reducing that =O to -H or that =O to -OH and -OH to H and dimethylamine that and it's done...
And also that sketch I did was wrong, that 3-carboned iodine chloro whatever should have only had 2 carbons.
But don't worry you're right Benzoin is going to be incredibly easier.... Just reducing that =O to -H or that =O to -OH and -OH to H and dimethylamine that and it's done...
Happyman
- Subordinate Wasp



- Posts: 122
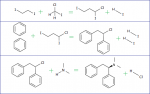
Whats the basic balanced equation for a akabori run with benzaldehyde & l-alanine. I tried it and got C7H6O(l)+2C3H7NO2(s)--->1C9H13NO(s)+2CO2(g)+1NH3(g)+1C2H4(g) Now that would be the coolest thing ever but I doubt it works that way. I wanna know so I can try and figure out what all the other ?-amino acids would do. Man. That looks more ridiculous every time I look at it.
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
Is this what you are looking for?
http://www.sciencemadness.org/scipics/akabori.png
Conversation here http://www.sciencemadness.org/talk/viewthread.php?tid=5979 might be of use to you.
http://www.sciencemadness.org/scipics/akabori.png
Conversation here http://www.sciencemadness.org/talk/viewthread.php?tid=5979 might be of use to you.
2bfrank
- Guest
Whats the basic balanced equation for a akabori run with benzaldehyde & l-alanine. I tried it and got C7H6O(l)+2C3H7NO2(s)--->1C9H13NO(s)+2CO2(g)+1NH3(g)+1C2H4(g) Now that would be the coolest thing ever but I doubt it works that way. I wanna know so I can try and figure out what all the other ?-amino acids would do. Man. That looks more ridiculous every time I look at it.
@happy man, on top of what Vesp suggested Naf1 and zz-zuchila went into that reaction, discussed the proposed mechanism, including ring formation, breakage, and in short is a very good read.. its in Naf1's thread called "tools for the synthetic chemist" it also covers other stuff, but hits pretty hard the akabori reacton etc.
Happyman
- Subordinate Wasp



- Posts: 122
Ripping from Seeman from http://www.sciencemadness.org/talk/viewthread.php?tid=5178
"And a quote from phenethyl_man from the now defunct the-hive.ws concerning a 4-bromo 2,5 DMBA for SWIY.
Quote:
A friend of mine told me this interesting story the other night.. Apparently he accidently broke his only condenser while disconnecting one of the hoses. Angered but not defeated he eyed the limited supply of chemicals at his disposal and here is what happenend:
In a round-bottomed flask equipped with magnetic stirring was added 250 mL of H2O, and 45 mL of 25% NaOH. 15 grams of hydroquinone was then added followed by 26mL of dimethyl sulfate. The flask was stirred at room temperature and after 15 minutes it was obviously no longer basic judging from the light color and flakes of hydroquinone floating around. At this point more 25% NaOH soln was added. In fact, he admits that too much base was added at this point which really hurt the yield in this reaction by slowing it down considerably. Anyhow, after another hour white crystals began to form out of the dark mixture and it took on the familiar smell of p-dimethoxybenzene. Stirring was continued for another 6 hrs, after which he became impatient and proceeded to vacuum filter the crystals on a buchner funnel and wash them with H2O. Yield: 11 grams (58%)
A solution of 10 g of potassium bromide in 250mL acetic acid was stirred on a ice/salt bath. When the solution was sufficiently cold, 5mL of 91% sulfuric acid was added slowly and the solution took on a light brown color. Over the course of the next 30 minutes, while maintaining the temperature at 5 degC, 8mL of 35% hydrogen peroxide was slowly added dropwise and subsequently the solution was allowed to stir for another 2 hrs. The soln was extracted w/2x75mL toluene and the extracts were washed w/50mL 5% NaOH, and then 50mL brine. The toluene was then removed in vacuo yielding a brown oil of presumably 1,4-dimethoxy-2-bromobenzene, which was used directly in the following reaction. Yield was not determined but appeared to him to be very good.
To an unknown amount of 1,4-dimethoxy-2-bromobenzene was added a soln. of 5 grams of glyoxylic acid monohydrate and 12 mL H2O. The solution was stirred vigorously on a ice/salt bath until a temperature of 5 degC was obtained and it was maintained at this temp throughout the subsequent reaction. Over the course of the next 30 minutes, 30mL 91% sulfuric acid was added dropwise; at this point the solution took on a dark black. The stirring was continued for the next 6 hours, the solution gradually taking on a light pink color with off-white crystals floating in the mixture. After completion of the reaction, the mixture was so viscous it could hardly be stirred. 100mL of cold water was then added, the mixture stirred for a short time longer and the crystals were vacuum filtered. The crystals were suspended in 100mL water and sufficent 25% aqueous NaOH was added until all of them dissolved into a dark brown solution. The solution was then extracted w/75mL toluene. The aqueous solution was once again cooled in an ice bath and ice-cold conc. HCl was added which cleared the solution and caused the crystals to precipitate back out and they were once again vacuum filtered and washed w/water. Yield: 20g of 4-bromo-2,5-dimethoxymandelic acid (86% from p-dimethoxybenzene)
To a solution of 40mL distilled water, and 20mL of 31.25% HCl was added a suspension of 20 grams of 4-bromo-2,5-dimethoxymandelic acid. The soln was cooled on an ice/salt bath to 5 degC and stirring was commenced. Into an addition funnel was added 4mL 70% nitric acid and 10mL water. The dilute HNO3 was added dropwise over about 15 minutes. The flask was removed from the ice bath and placed into a water bath maintained at 50 degC. It was heated and stirred at this temperature for about 1 hr. The flask was cooled to room temperature and an ice-cold solution of 60mL 25% NaOH was added which caused the unreacted acid to dissolve into the mixture. The remaining crystals were vacuum filtered and recrystallized from methanol. Yield 12.5g (74%).
Overall yield: 12.5g 4-bromo-2,5-dimethoxybenzaldehyde from 15g hydroquinone (37.5%)"
How would replacing potassium fluoride for potassium bromide do? Could you pull off 4-fluoro-2,5-dimethoxybenzaldehyde? Sorry if I missed an obvious error, I wanted to put this in tonight and I've only given it about 10 minutes of thought.
"And a quote from phenethyl_man from the now defunct the-hive.ws concerning a 4-bromo 2,5 DMBA for SWIY.
Quote:
A friend of mine told me this interesting story the other night.. Apparently he accidently broke his only condenser while disconnecting one of the hoses. Angered but not defeated he eyed the limited supply of chemicals at his disposal and here is what happenend:
In a round-bottomed flask equipped with magnetic stirring was added 250 mL of H2O, and 45 mL of 25% NaOH. 15 grams of hydroquinone was then added followed by 26mL of dimethyl sulfate. The flask was stirred at room temperature and after 15 minutes it was obviously no longer basic judging from the light color and flakes of hydroquinone floating around. At this point more 25% NaOH soln was added. In fact, he admits that too much base was added at this point which really hurt the yield in this reaction by slowing it down considerably. Anyhow, after another hour white crystals began to form out of the dark mixture and it took on the familiar smell of p-dimethoxybenzene. Stirring was continued for another 6 hrs, after which he became impatient and proceeded to vacuum filter the crystals on a buchner funnel and wash them with H2O. Yield: 11 grams (58%)
A solution of 10 g of potassium bromide in 250mL acetic acid was stirred on a ice/salt bath. When the solution was sufficiently cold, 5mL of 91% sulfuric acid was added slowly and the solution took on a light brown color. Over the course of the next 30 minutes, while maintaining the temperature at 5 degC, 8mL of 35% hydrogen peroxide was slowly added dropwise and subsequently the solution was allowed to stir for another 2 hrs. The soln was extracted w/2x75mL toluene and the extracts were washed w/50mL 5% NaOH, and then 50mL brine. The toluene was then removed in vacuo yielding a brown oil of presumably 1,4-dimethoxy-2-bromobenzene, which was used directly in the following reaction. Yield was not determined but appeared to him to be very good.
To an unknown amount of 1,4-dimethoxy-2-bromobenzene was added a soln. of 5 grams of glyoxylic acid monohydrate and 12 mL H2O. The solution was stirred vigorously on a ice/salt bath until a temperature of 5 degC was obtained and it was maintained at this temp throughout the subsequent reaction. Over the course of the next 30 minutes, 30mL 91% sulfuric acid was added dropwise; at this point the solution took on a dark black. The stirring was continued for the next 6 hours, the solution gradually taking on a light pink color with off-white crystals floating in the mixture. After completion of the reaction, the mixture was so viscous it could hardly be stirred. 100mL of cold water was then added, the mixture stirred for a short time longer and the crystals were vacuum filtered. The crystals were suspended in 100mL water and sufficent 25% aqueous NaOH was added until all of them dissolved into a dark brown solution. The solution was then extracted w/75mL toluene. The aqueous solution was once again cooled in an ice bath and ice-cold conc. HCl was added which cleared the solution and caused the crystals to precipitate back out and they were once again vacuum filtered and washed w/water. Yield: 20g of 4-bromo-2,5-dimethoxymandelic acid (86% from p-dimethoxybenzene)
To a solution of 40mL distilled water, and 20mL of 31.25% HCl was added a suspension of 20 grams of 4-bromo-2,5-dimethoxymandelic acid. The soln was cooled on an ice/salt bath to 5 degC and stirring was commenced. Into an addition funnel was added 4mL 70% nitric acid and 10mL water. The dilute HNO3 was added dropwise over about 15 minutes. The flask was removed from the ice bath and placed into a water bath maintained at 50 degC. It was heated and stirred at this temperature for about 1 hr. The flask was cooled to room temperature and an ice-cold solution of 60mL 25% NaOH was added which caused the unreacted acid to dissolve into the mixture. The remaining crystals were vacuum filtered and recrystallized from methanol. Yield 12.5g (74%).
Overall yield: 12.5g 4-bromo-2,5-dimethoxybenzaldehyde from 15g hydroquinone (37.5%)"
How would replacing potassium fluoride for potassium bromide do? Could you pull off 4-fluoro-2,5-dimethoxybenzaldehyde? Sorry if I missed an obvious error, I wanted to put this in tonight and I've only given it about 10 minutes of thought.
Douchermann
- Dominant Queen




- Posts: 397
I don't believe H2O2 will oxidize the fluorine out of potassium fluoride. It'll more than likely just leave you with HF. If it does happen to oxidize it out... watch out hahaha.
Vesp
- Administrator
- Foundress Queen





- Posts: 3,130
fluorine is more electronegative then oxygen, while chlorine, bromine, and iodine are less. So H2O2 can't oxidize fluoride to fluorine.